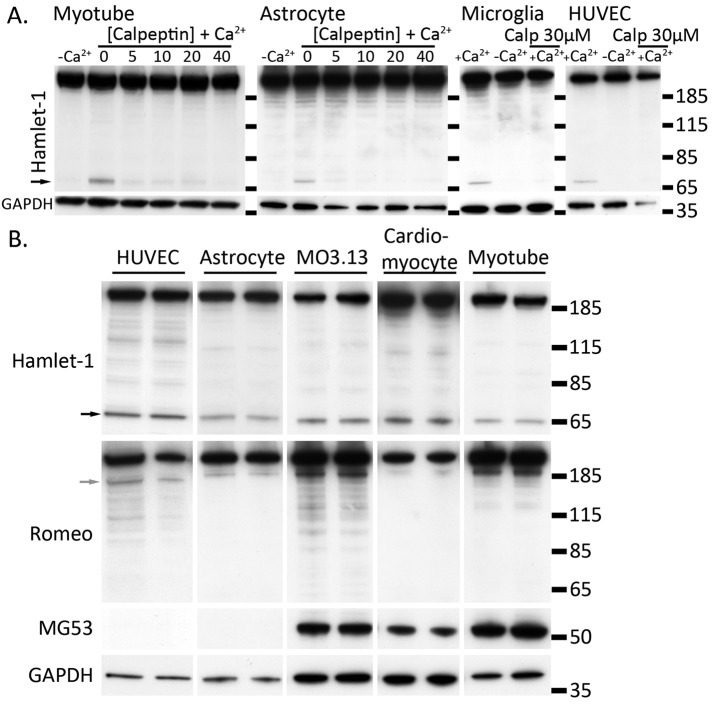
FIGURE 4:
Dysferlin is cleaved in multiple cells types independent of MG53. (A, B) Injury-activated formation of mini-dysferlinC72 is calcium dependent and blocked by calpeptin and occurs in multiple cell lineages. (A) Cells were cultured to confluence and damaged by scraping in the presence or absence of Ca2+ or the presence of Ca2+ plus the calpain inhibitor calpeptin (Calp). Cell pellets were lysed in RIPA, and 10 μg of protein was separated by SDS–PAGE and transferred onto PVDF membrane. One PVDF membrane was probed with Hamlet-1, which detects the dysferlin C-terminus and mini-dysferlinC72 (black arrowhead). The duplicate PVDF membrane was probed with Romeo, detecting the dysferlin N-terminus and corresponding cleaved N-terminal fragment (gray arrowhead). Membranes were reprobed with anti-MG53 or anti-GAPDH to show equal loading. (B) Mouse astrocytes and human umbilical vein endothelial cells do not express MG53, and thus formation of mini-dysferlinC72 occurs independently of MG53.