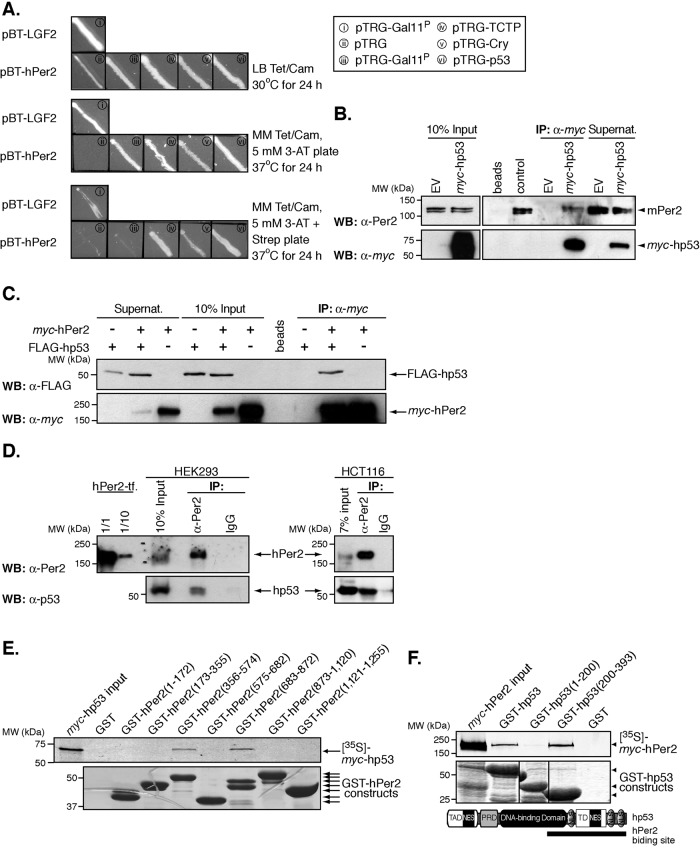
FIGURE 1:
The circadian factor hPer2 interacts with hp53. (A) Two-hybrid protein–protein interaction between hPer2, hp53, TCTP, and Cry. Each pair of plasmids—i) pBT-LGF2 + pTRG-Gal11P, ii) pBT-hPer2 + pTRG, iii) pBT-hPer2 + pTRG-Gal11P, iv) pBT-hPer2 + pTRG-TCTP, v) pBT-hPer2 + pTRG-Cry, and vi) pTRG-hp53—was grown on nonselective medium plus antibiotics (LB tetracycline [Tet]/chloramphenicol [Cam]) and later patched on both selective screening minimum medium (MM Tet/Cam/5 mM 3-AT) and dual-selective minimum medium containing MM Tet/Cam/5 mM 3-AT/streptomycin (Strep). Positive controls were i and v, whereas ii and iii were negative. (B) Pellets from CHO cells transfected with pCS2+myc-hp53 were lysed in 25 mM Tris-phosphate pH 7.8, 2 mM dithiothreitol, 2 mM 1,2-diaminocyclohexane-N,N,N′,N′-tetraacetic acid, 10% glycerol, and 1% Triton X-100, and extracts (∼300 μg) were incubated with α-myc beads. Endogenous mPer2 and recombinantly expressed hp53 were detected by using either α-Per2 (top) or -myc antibodies (bottom). Control indicates 20 μg of total extract. (C) Samples from CHO cells cotransfected with pCS2+myc-hPer2 and pCS2+FLAG-hp53 were immunoprecipitated using α-myc beads and immunoblotted using α-FLAG (top) and -myc antibodies (bottom). (D) One milligram of HEK293 and HCT116 extracts was incubated with either α-Per2 or IgG. Complexes were immunoprecipitated using protein A beads and immunoblotted for endogenous proteins using α-Per2 (top) and -p53 antibodies (bottom). For the positive control, HEK293 cells were transfected with pCS2+hPer2 (hPer2-tf), and total cell extracts (20 μg [1/1] and 2 μg [1/10]) were loaded. (E) Recombinant GST-tagged fragments of hPer2 were purified using affinity chromatography, and bound beads were incubated with 35S-labeled myc-hp53 and assayed for binding as described in the Supplemental Material. Bound complexes were visualized by Coomassie staining (bottom) and the radiolabeled protein detected by autoradiography (top). (F) Mapping of hPer2-binding regions in hp53. Schematic representation of hp53 architecture (393 residues), including the transactivation (TAD; residues 1–42), proline-rich (PRD; residues 61–92), DNA-binding (residues 101–300), and tetramerization domains (TD; residues 326–356). The binding site in hPer2 is indicated with a solid line. Bead-bound GST-hp53 and recombinant proteins were incubated with [35S]myc-hPer2 and analyzed for complex formation as described. In all cases, GST beads were used as a negative control; beads, matrix sample with no antibody bound; EV, empty vector; Supernat, supernatant fraction after immunoprecipitation (IP); transf, transfected cells; B–F show immunoblot data from a single experiment that was repeated three times with similar results.