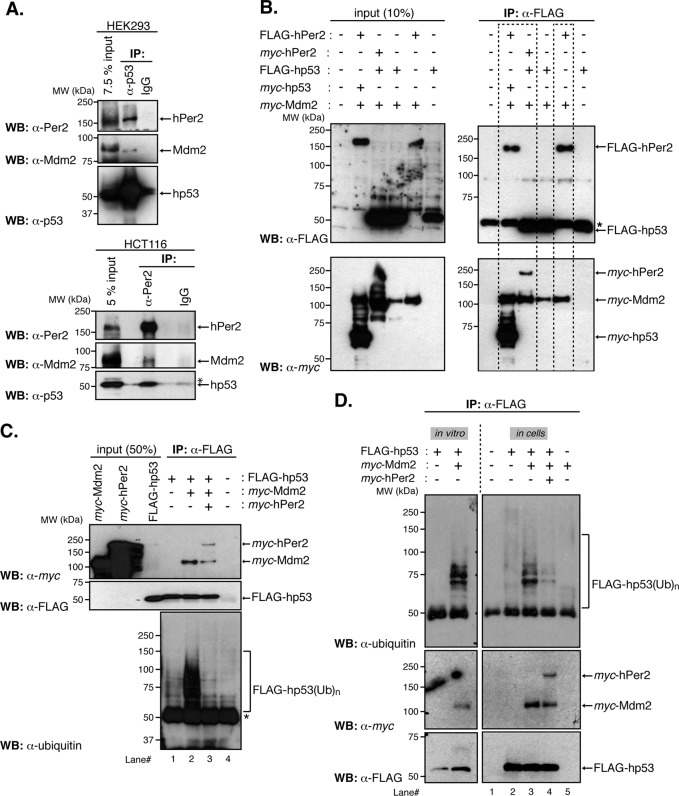
FIGURE 2:
The hPer2 protein forms a ternary complex with hp53 and Mdm2, controlling the extent of hp53 ubiquitination. (A) HEK293 and HCT116 protein extracts (1 and 2 mg, respectively) were incubated with either α-p53 or α-Per2, respectively, and protein A beads. Rabbit IgG was used as a negative control. Immunoprecipitated complexes were analyzed for the presence of hPer2, hp53, and Mdm2 using specific antibodies (top for HEK293 and bottom for HCT116). Asterisk indicates a nonspecific signal. (B) HEK293 cells were transfected with pCS2+myc-Mdm2, pCS2+myc-hp53, pCS2+FLAG-hp53, pCS2+FLAG-hPer2, pCS2+myc-hPer2, or a combination of plasmids and complexes immunoprecipitated using α-FLAG–coupled beads. Complex components were identified by immunoblotting using α-FLAG and α-myc antibodies (right top and bottom). Input amounts were monitored in cell lysates (20 μg) and shown in the left top and bottom. Results similar to those presented were observed in two independent experiments. (C) In vitro–synthesized myc-hPer2 and FLAG-hp53 proteins were preincubated before the addition of myc-Mdm2 (ratio 1:2:5: FLAG-hp53:myc-Mdm2:myc-hPer2). Samples were then subjected to in vitro ubiquitination, followed by immunoprecipitation of hp53-bound proteins using α-FLAG antibody and protein A beads. Bound proteins were detected by immunoblotting and are indicated with arrows (top and middle). FLAG-hp53(Ub)n forms of hp53 were detected using α-ubiquitin antibody (bottom). Asterisk indicates IgG heavy chain. (D) HEK293 cells were transfected with pCS2+myc-Mdm2, pCS2+myc-hPer2, pCS2+FLAG-hp53, or a combination of plasmids and collected 12 h after treatment with 10 μM MG132. Cell lysates (100 μg) were incubated with α-FLAG and protein A beads and hp53-ubiquitinated complexes (FLAG-hp53(Ub)n) detected using α-ubiquitin antibody (top right). Bound proteins were visualized by immunoblotting using α-myc and α-FLAG antibodies (middle and lower right). An in vitro ubiquitination reaction was performed as described in C and is shown as control and for comparison purposes with the “in cells” result (left). Brackets denote ubiquitinated hp53. A, C, and D show immunoblot data from a single experiment that was repeated three times with similar results.