FIGURE 6:
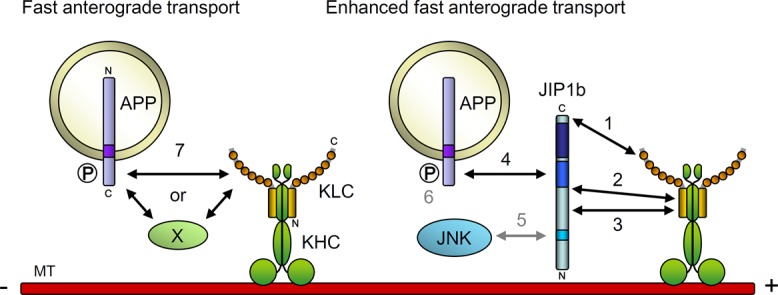
Functional interaction between APP, JIP1b, and KLC of kinesin-1. Possible regulation of APP cargo transport by protein interactions is shown schematically. 1) Association of JIP1b C11 to the TPR motif of KLC1 is required for the enhanced fast velocity of anterograde transport of APP cargo. 2) Interaction of JIP1b465-483 with KLC1 N200 regulates the association of JIP1b C11 to the TPR motif of KLC1 and is thus essential for the enhanced fast velocity of anterograde transport of APP cargo. This interaction may also be involved in the decrease of the stationary APP cargo. 3) Interaction of JIP1b370-402 with KLC1 N200 contributes to the stable and higher-frequency anterograde transport of APP cargo. 4) Interaction of the JIP1b PI/PTB domain with the APP cytoplasmic NPTY motif is essential for the enhanced fast velocity and higher frequency of anterograde transport of APP cargo. 5) Interaction of JIP1b JBD with JNK is not involved in the enhanced fast velocity of anterograde transport of APP cargo. 6) Phosphorylation of APP at cytoplasmic Thr668 is not involved in the efficient APP anterograde transport by kinesin-1. 7) APP cargoes may interact with kinesin-1 independently of JIP1 to be transported anterogradely with slower velocity. An unknown factor, X, may mediate the interaction of APP with kinesin-1, or APP can directly associate with kinesin-1 in the absence of JIP1.
