Abstract
Physiologically accurate mouse models of cancer are critical in the pre-clinical development of novel cancer therapies. However, current standardized animal-housing temperatures elicit chronic cold-associated stress in mice, which is further increased in the presence of tumor. This cold-stress significantly impacts experimental outcomes. Data from our lab and others suggests standard housing fundamentally alters murine physiology, and this can produce altered immune baselines in tumor and other disease models. Researchers may thus underestimate the efficacy of therapies that are benefitted by immune responses. A potential mediator, norepinephrine, also underlies stress pathways common in mice and humans. Therefore, research into mechanisms connecting cold-stress and norepinephrine signaling with immune-depression in mice could highlight new combination therapies for humans to simultaneously target stress while stimulating anti-tumor immunity.
Keywords: cancer, immunology, murine model, norepinephrine, stress, T cells, thermoregulation
Introduction
Cancer has posed a burden on human society since ancient times. Over the past century, cancer therapies have evolved through medical and scientific advancement to include surgery, radiation, chemotherapy, as well as specialized biological inhibitors and other treatments that target genetic facets of cancer. For both old and new interventions, efficacy varies greatly, depending on tumor type and individual patient characteristics. It is now clear that a number of these therapeutic strategies are greatly benefitted by anti-tumor immune responses generated as side-effects of therapy [1, 2]. This highlights the value of the immune system in protecting patients from aggressive disease and cancer recurrence and also prompts the search for ways to enhance immune responses in conjunction with other therapies. Indeed, cancer immunotherapies were heralded as the major breakthrough of 2013 [3].
Much of our pre-clinical data evaluating potential new therapeutic combinations relies on the use of animal models. Mice in particular are highly popular in the field of cancer immunology, offering a number of benefits to the researcher. Mice are generally less expensive, easier to maintain, and have relatively short generation times compared to other mammals [4, 5]. In addition to a panoply of murine specific antibodies and reagents for analysis, a large number of mouse strains have been generated as important tools in cancer immunology [6]. These include transgenic expression of cancer oncogenes, selective knock-out of tumor suppressors, as well as specific knock-out of a number of immunologically relevant receptors and ligands for study. Despite their wide use as surrogates for the study of human cancer development, significant differences exist between mice and humans, particularly in the area of metabolism [7] and thermoregulation [4]. These differences often do not receive sufficient attention from researchers using murine models to study tumor growth and anti-tumor immunity.
This review addresses current problems and paradigms in the area of cancer immunology research, with a focus on the striking role ambient temperature plays in shaping experimental outcomes in our pre-clinical mouse models. Emerging data demonstrate that ambient temperature has significant effects on murine biology and specifically on anti-cancer immune responses by mice. We propose that greater awareness of these effects will contribute to enhanced design of animal models for cancer. These observations can also be co-opted as excellent models for understanding the role of stress in tumor development.
Mammals use multiple approaches to adapt to ambient temperature
Mammals are defined as endotherms, or animals that maintain a particular metabolically favorable temperature. For humans and mice, this internal temperature is approximately 37°C. In order to maintain this temperature in a range of external environments, they have evolved a number of thermo-regulatory mechanisms [8]. These mechanisms can be divided into two main categories: conscious behaviors and non-conscious physiological responses. Warming behaviors include burrowing, huddling, increasing activity, and, for humans, adding additional layers of clothes. Involuntary, physiological methods of conserving heat include constricting blood flow (vasoconstriction), raising the hair on the skin surface to form a more insulating area (piloerection), as well as adopting a hunched posture to reduce the amount of exposed surface area [9]. In the face of continued cold, mammals have the ability to increase their own heat production, or thermogenesis. Thermogenesis occurs via two major pathways, shivering and nonshivering. Both represent increases in the mammalian basal metabolic rate and are controlled by a complicated network of signaling events. Generally, shivering thermogenesis is a short-term response and is rapidly turned off once the mammal is returned to a warm environment. Non-shivering thermogenesis, associated with altered metabolism, hormonal signaling, and activity in the brown fat pads, is considered to have more long term consequences but is more difficult to measure [9].
Thermoneutrality is defined as the temperature at which mammals need to exert the least amount of energy to maintain their normal body temperature (Figure 1). This temperature depends on the rate at which heat dissipates from the animal, controlled by a variety of factors including insulation (fur, skin thickness, and clothes), body composition, as well as the ratio of surface area to volume. Mouse bodies have a higher surface to volume ratio than humans resulting in greater opportunity for heat loss to the external environment [10]. The Guide for the Care and Use of Laboratory Animals recognizes that mice exhibit a preference for temperatures above 26°C [11], and this figure is in line with several calculations for the expected thermoneutral range of mice [11–16]. However, the Guide’s mandates on housing temperatures do not reflect this preference, as it requires that mice be maintained between 20 and 26°C [11]. Housing temperatures may be even lower in international research facilities.
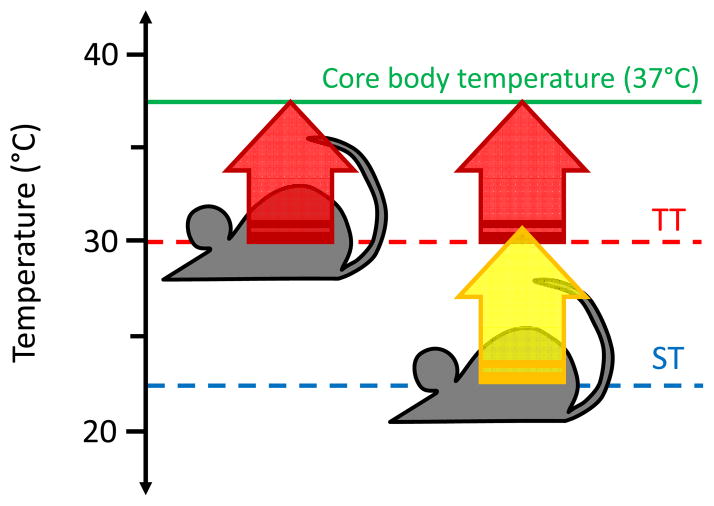
Figure 1.
Mice at lower ambient temperatures require increased rates of metabolism and thermogenesis. The normal core body temperature of mice is approximately 37°C (green line), as for most mammals. Mice housed at their thermoneutral temperature (~30°C, TT, red dashed line) are able to meet this temperature using only their basal metabolic rate (BMR) for heat production (red arrow). Mice housed under standard temperature conditions (~22°C, ST, red dashed line) cannot rely solely on their BMR, and thus a number of adaptive thermogenic pathways are turned on to maintain normal body temperature (yellow arrow).
Mice maintained at standard temperatures have the same core body temperature as mice in thermoneutral environments; however, they display several outward signs of ‘feeling cold.’ The options mice have for dealing with this cold are limited. Mice cannot add layers of clothes, but when given additional insulating materials, standard temperature mice build more elaborate nests [13, 14]. The shoebox housing commonly used for mouse research also greatly limits the space for mice to increase warming physical activity [7]. Thus, mice at standard housing spend more time huddling in groups compared to their thermoneutral counterparts [5, 13, 14]. Mice in standard housing do have unlimited access to food; it has been observed that mice in cold temperatures consume significantly more calories than mice at thermoneutrality [17, 18]. These observations underline the fact that standard temperature-housed mice experience chronic cold-induced stress. Additionally, we have observed increased levels of the hormone norepinephrine (NE) in these animals which is even further increased in tumor bearing animals (Eng et al., manuscript in preparation). NE is classically associated with inducing thermogenesis [9]; it has also been implicated in a number of human stress pathways and is a key measure of stress in these animals.
Cold stress significantly impacts murine physiology
As mentioned above, ambient temperature determines the level of metabolism in mammals that is required to maintain core body temperatures. Adaptations to chronic cold temperatures can have a profound effect on rodent physiology. By comparing mice housed at standard temperatures (20–26°C, ST) to mice housed at their thermoneutral temperature (28–32°C, TT), we and others have identified a number of significant differences that impact experimental models. Examples include basic differences in growth and development [19–22], altered sleep and activity patterns [23–27], altered hormonal balance [28–30], as well as altered metabolism and susceptibility to metabolic syndrome [31–35]. Indeed, an excellent example of the profound effect of chronic cold stress on murine physiology and the potential impact on experimental outcomes has been in the field of metabolism. UCP1, a gene involved in thermoregulation and implicated in metabolic pathways underlying human obesity [36, 37], was knocked out in mice, but this resulted in no overt obese phenotype under standard housing. It was not until mice were housed at thermoneutrality that the obesogenic nature of this deletion was revealed [31]. Thus, the means mice rely upon to cope with chronically reduced ambient temperatures in housing facilities fundamentally alters their biology. Other biological processes affected by ambient temperature are summarized in Table 1.
Table 1.
Differences between animals housed at Standard Temperature (ST) and Thermoneutral Temperature (TT)
| ST | TT | |
|---|---|---|
| Core body temperature [4] | = | = |
| Metabolism [87] | + | − |
| Heart rate [28] | + | − |
| Food intake [17, 18] | + | − |
| Weight [4, 15] | + | − |
| Serum lipids [88] | + | − |
| Norepinephrine production [88] | + | − |
Relative expression of the indicated measures is shown as = (equivalent), + (increased), or − (decreased) between ST and TT conditions.
Not only does ambient temperature affect homeostatic properties of murine development, it also alters the ability of mice to respond to additional stressors. Though similar at baseline, our lab has observed that mice housed at ST and TT experience differential expression of heat shock factor-1 (HSF-1) in numerous organs upon short term exposure to hyperthermic temperatures well above their thermoneutral zone (resulting in mild whole body hyperthermia) (Reed et al, manuscript pending revision). Concurrently, expression of the heat shock proteins HSP70, HSP90, and HSP110 differed significantly between ST and TT mice following this therapy. In addition to their role in temperature responses, heat shock proteins maintain protein stability and fundamental cellular biology in the face of hypoglycemia, hypoxia, and other cellular stressors. Such striking differences in heat shock protein expression suggests that mice housed at different ambient temperatures would have differential susceptibilities to a number of additional stressful interventions, including surgery, radiation, and chemotherapy, as well as models of ischemia/reperfusion injury and other conditions.
In addition to the differences in basic biology discussed above, differences in immune phenomena between ST and TT housed animals have been observed. Karp has published an excellent review of the effects of cool ambient temperatures on several facets of murine immune responses to infectious disease and other pathologies with immune components such as atherosclerosis and cystic fibrosis [5]. Generally, mice maintained under standard housing temperatures have altered susceptibilities to infectious diseases and often do not develop pathological symptoms associated with immune function in human patients. These findings have should be of concern to researchers who study immunological problems in murine models. Given the current appreciation of immunotherapy as a great advance in cancer treatment for patients [3], the effects of cold stress in murine cancer models deserves more study.
Chronic cold stress profoundly impacts cancer models
Despite the profound effects of chronic cold stress on murine physiology, data for the cancer setting have only recently been published. Kokolus et al demonstrated that mice under chronic cold stress due to standard temperature housing (22°C, ST) are more susceptible to tumor growth compared to their non-stressed thermoneutral counterparts (30°C, TT) [15]. Transplantable, orthotopic tumors grow with faster kinetics in ST hosts and also produce more metastatic lesions. Additionally, exposing mice to the carcinogen methylcholanthrene (MCA) resulted in tumor development in 100% of mice at ST but only 20% of mice at TT after 140 days. Thus, it is clear that standard housed, cold-stressed mice, used routinely for cancer studies, suffer from increased tumor burdens compared to warmer animals.
Tumor growth is dependent on a wide array of variables that could be impacted by ambient temperature as described above. Interestingly, Kokolus et al. identified endogenous immune responses as an especially strong determining factor for the observed difference in tumor growth rates. Challenging immune-compromised mice with tumors resulted in similar tumor growth rates between the two temperature conditions. Additionally, depletion of immune effector T cells, specifically CD8+ T cells, abolished the delay in tumor growth observed at TT. Thus, despite other potential factors in the cold-stressed mice that could contribute to tumor growth, an intact immune response plays a major role in reducing tumor burdens in non-stressed animals.
Intriguingly, gross immune populations from naïve (i.e. non-tumor bearing) mice at ST versus TT are comparable [15], suggesting that homeostatic immune development is not affected by temperature. However, under the added stress of tumor burden, the predominant immune populations are strikingly different between the two temperatures. ST mice have higher numbers of suppressive regulatory T cells (T regs) within the tumor, while TT mice have a large number of infiltrating CD8 T cells that appear to be functionally active. The size of the spleen increases drastically in tumor-bearing mice, but the size is larger for ST mice compared to TT mice [15, 38], and the difference is largely due to increased numbers of myeloid cells. These cells include macrophages, dendritic cells, and myeloid-derived suppressor cells. Comparing these populations from mice at TT, myeloid cells from ST mice are less mature and exhibit an overall decreased ability to activate T cells [38]. Immature, suppressive myeloid cells have been strongly implicated in promoting tumor growth in a number of cancer settings [39, 40]. Mechanisms underlying this difference in myeloid populations between ST and TT mice deserve more research as a major target for cancer therapies.
Adrenergic signaling likely contributes to stress-mediated immune dysfunction in cancer models
We propose here a model to highlight the systemic effects of stress on immune responses, with a focus on the cancer setting (Figure 2). As discussed above, norepinephrine (NE) is the primary signaling hormone associated with adaptive thermogenesis. NE has also been strongly associated with cancer development [41–45]. The primary source of systemic NE, the sympathetic nervous system (SNS), innervates vital immune organs including primary lymphoid tissues such as the bone marrow and secondary, peripheral lymphoid tissues such as the spleen and lymph nodes [46]. Thus, NE signaling via the SNS in response to ambient temperature potentially modulates anti-tumor immune responses at all stages, from progenitor development in the bone marrow, inhibition or suppression of antigen presentation and T cell priming in the lymphoid organs, as well as responsiveness of lymphocytes at the tumor site itself and in other peripheral tissues.
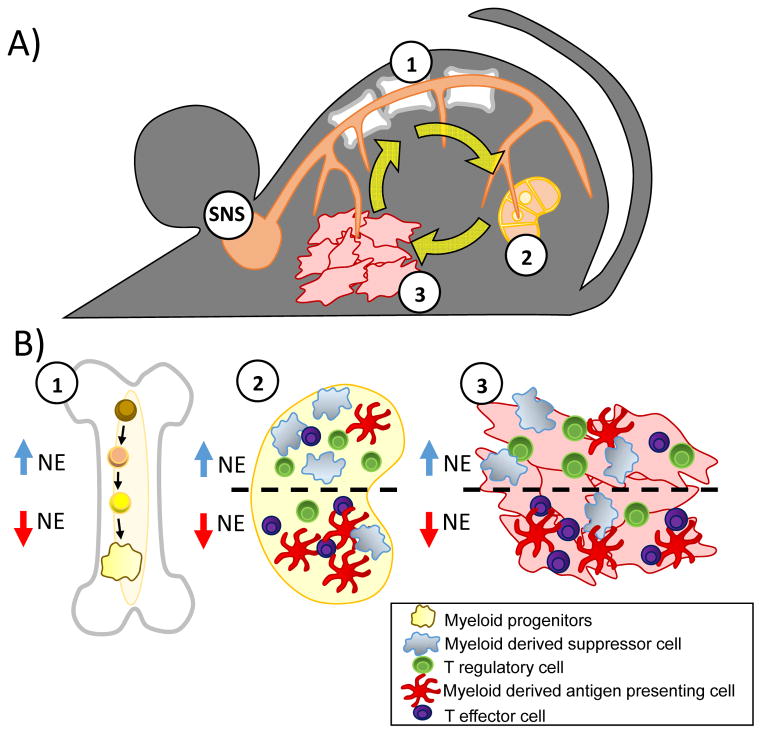
Figure 2.
Chronic stress-associated norepinephrine systemically alters immune responses in the tumor bearing mouse. A: Stress activates the sympathetic nervous system (SN) to produce norepinephrine (NE). NE potentially targets three major organs of interest in cancer research: (1) primary lymphoid tissue - bone marrow, (2) secondary/peripheral lymphoid tissues - lymph nodes, spleen, and (3) the tumor microenvironment. B: Potential cellular targets at each of these sites are highlighted: (1) hematopoietic progenitor cells in the bone marrow, (2) myeloid cells and lymphocytes in peripheral lymphoid tissues, (3) tumor cells as well as tumor-associated myeloid cells and lymphocytes. With increasing NE, the balance of suppressive to effector cells within the tumor, spleen, and lymph nodes shifts toward pro-tumorigenic populations. NE signaling during both myeloid and lymphoid development and upon fully differentiated cells likely contributes to this altered immune environment.
Bone marrow progenitor cells express adrenergic receptors [47], rendering them capable of responding to NE signaling [46]. Social stress and thermal injury have been associated with alterations in lympho- and myelopoiesis which could be blocked by antagonists of adrenergic signaling [48–50]. However, administration of NE alone was not enough to elicit these changes. Thus, NE, in the presence of other factors such as tumor derived G-CSF or GM-CSF, potentially contributes to granulocytic skewing of the myeloid compartment giving rise to suppressive cell types. The exact mechanism by which NE signaling contributes to lineage commitment remains to be elucidated. In addition to effects on cellular development, NE differentially promotes myeloid or lymphoid egress from bone marrow into the periphery [51–53]. Again, the exact signaling pathways behind these observations remain to be explored, and this phenomenon appears to also be dependent on other factors in the bone marrow. Taken together, the production of NE by sympathetic nerves in response to stress potentially targets immune cells directly at their site of manufacture to alter lineage skewing and deployment to peripheral tissues. This is likely modulated by tumor derived factors.
The secondary lymphoid organs, comprised of the lymph nodes and spleen, are sites of training, or ‘priming,’ naive immune cells to develop effective immune responses against detected threats. It has long been known that these organs are heavily innervated by sympathetic neurons [46]. NE signaling through β-adrenergic receptors on antigen presenting cells can dramatically alter their cytokine production, suppressing inflammatory cytokines such as IL-12 and TNFα while promoting production of anti-inflammatory cytokines such as IL-10 [46, 54]. Cytokine milieu at the site of priming determines the type of cellular immunity elicited; overall, NE appears to promote a ‘Th2’ phenotype more typically associated with anti-helminthic or allergic responses, as opposed to ‘Th1’ responses which are considered to be more protective in the cancer setting. Such cytokine imbalances may also contribute to enhanced regulatory T cell responses. Thus, NE at the site of immune priming may skew potential effector cells away from an effective anti-tumor phenotype toward pro-tumor immune responses.
It is clear that NE within the tumor microenvironment also contributes to disease progression [44, 55]. Numerous sources of intratumoral NE have been identified and may be tumor specific; some tumors have been shown to trigger neural invasion for direct interaction with the SNS [56]. Additionally, tumor-associated macrophages [57–60], lymphocytes [61], or other stromal components [62] can also contribute to local NE production. It is also clear that inflammatory cytokines form a feedback loop with the SNS to regulate catecholamine production [63–65]. Regardless of source, NE signaling through β-adrenergic receptors at the tumor site can have diverse immunologically relevant effects: 1) T regulatory cells [66, 67], myeloid derived suppressor cells, or macrophages [68] experience enhanced suppressor function, 2) effector T cells experience reduced proliferation and production of survival cytokine IL-2 and effector cytokines IFNγ and TNFα [66,69,70], 3) signaling on tumor cells can directly decrease the expression of immune-stimulatory molecules [71]. These effects are primarily mediated by downstream increases in intracellular cyclic AMP and activation of PKA. It is important to note that NE can also stimulate adrenergic receptor positive tumor cells directly to promote tumor growth and metastasis via immune-independent pathways [41, 71]. Thus, targeting of NE signaling can have a global benefit on boosting immune responses, but can also be developed as a targeted therapy for patients with tumors that are adrenergic receptor positive.
Comparing animals from varied housing conditions could improve disease models
There is considerable debate in the field regarding whether the warm housing environment more or less accurately represents the human condition. As others have pointed out, humans routinely live in environments below their thermoneutral temperature [72]. Humans, however, have developed a number of tools to manipulate their environment, reducing the need for purely physiological adaptations to reduce cold- (or hot-) stress. This level of environmental control is unavailable to laboratory mice. Whether ambient temperature impacts human cancer incidence or mortality has not been determined. This is due to confounding factors such as infection rates, UV exposure, developmental status, and other regional variations that make such investigations difficult. However, we do not believe the observations discussed above are limited to only cold-stress. Human cancer patients face a variety of other environmental and psychological stressors over the course of pre-clinical tumor development, clinical diagnosis, and therapy. Importantly, in both mice and humans, such stressors are also largely associated with increased NE production including: social isolation [55, 73, 74], social disruption [75, 76], depression [77, 78], burn injury [50, 79] and others [80]. Standard housing may thus be an excellent model for certain patients that are experiencing long-term chronic stress or have otherwise elevated levels of NE. Indeed, epidemiological evidence has already shown that patients on antagonists of NE signaling at the time of their chemotherapy had improved disease outcomes for breast cancer as well as melanoma [81]. Such antagonists, termed ‘beta-blockers’ for their specific effects of inhibiting NE signaling through beta adrenergic receptors, are commonly prescribed to patients for treatment of high blood pressure. There is a great need for prospective studies and/or randomized trials to investigate the potential benefits to combining cancer immunotherapy with such beta-blockers or other pharmaceuticals that intervene in stress response pathways.
Interestingly, tumor growth itself represents an additional metabolic stress. This is reflected in the observation that tumor bearing mice prefer to be housed at temperatures even warmer than 30°C [15], and tumor burden increases NE levels in mice under both ST and TT housing (Eng et al., manuscript in preparation). Immune responses are considered to be metabolically expensive events [82, 83]. Under conditions of metabolic stress, immune function experiences trade-offs to preserve other physiologic processes [84]. Thus, hormones such as NE may serve as regulators of immune responses to preserve metabolic function for survival in the face of environmental stress. Intriguingly, similar to tumor-bearing mice, cancer patients frequently report feeling cold or experiencing other temperature-related symptoms [85]. Thus, changes in metabolism and thermoregulation can also be associated with cancer development in humans.
Assessment across a number of infectious disease models has shown that mice at thermoneutrality tend to more closely recapitulate human pathologies associated with active immune responses [5]. Cancer models that rely solely on mice in standard housing may not fully reflect immune effects over the course of tumor development. Non-stressed TT mice have highly functioning immune responses, but still only suppress, rather than outright reject, most tumors [15]. The tumors that grow out in mice at TT may offer insight into novel immune escape mechanisms to be targeted in human patients. Also, since TT tumors grow at a slower rate and are under greater selective pressure, they may better reflect human tumors which have also undergone strong selective pressure before becoming clinically detectable. It will be very important to determine whether these tumors are more or less refractory to common cancer therapeutics, which may help explain how a number of drugs that were promising at suppressing tumor growth in mice have been less than successful in the clinic.
We suggest that mice should be evaluated under more than one ambient temperature to more fully understand the range of their ability to respond to challenges that model human disease. Several strategies are available for this effort, including the use of environmentally controlled incubators for maintaining mice at various ambient temperatures, including thermoneutrality, as described in the studies reported here. However, this may not always be practical. Simply introducing more bedding material to allow mice greater control over their own thermal comfort greatly reduces stress experienced by mice in standard housing [17,86]. Modeling diseases with or without environmental manipulation requires very little additional intervention and will aid the assessment of stress signaling in these disease pathways.
Conclusions and outlook
Chronic stress in murine models has limited our ability to achieve a complete understanding of potential therapeutic outcomes. Given that positive pre-clinical data are necessary to justify the expense of seeing a new therapy through clinical trials, this may have resulted in a number of missed opportunities for immune-associated therapies that might have been effective if tested in non-stressed animals with highly functioning immune systems. Appreciation of the role of patients’ endogenous immune responses to cancer therapies is a developing phenomenon, and there remain a number of questions regarding the best way to stimulate this protection. Future research into novel cancer therapeutics would be benefitted by simple interventions that reduce stress in laboratory mice to allow for more robust immune outcomes. It will also be important to consider ambient temperature in development of new spontaneous cancer models; by comparing chronic mild cold-stress to reduced stress animals, investigators will be able to identify differences between positive and negative stress states, immune responses, and other processes active during disease progression that may more or less reflect human outcomes. This research should be significantly motivated by the centrality of norepinephrine signaling in multiple human stress pathways and the potential impact of NE on anti-tumor responses. Indeed, human epidemiologic data has already shown that blocking NE signaling can protect patients from tumor recurrence and metastasis. Surely the use of these and other drugs that target stress responses in combination with immunogenic therapies deserves further study.
Table 2.
Alterations in immune responses between animals housed at Standard Temperature (ST) and Thermoneutral Temperature (TT)
| ST | TT | |
|---|---|---|
| % CD3+ T cells [15] | − | + |
| % CD8+ T cells [15] | − | + |
| % Treg cells [15] | + | − |
| # Myeloid cells [15, 38] | + | − |
| # Myeloid-Derived Suppressor Cells [15, 38] | + | − |
| # Dendritic Cells (DC) [15, 38] | + | − |
| % MHC II+CD86+ DCs [38] | = | = |
| DC-T cell Activation [38] | − | + |
| Alternatively activated macrophages [60] | + | − |
| Norepinephrine production by macrophages [60] | + | − |
Relative expression of the indicated measures is shown as = (equivalent), + (increased), or − (decreased) between ST and TT conditions.
Abbreviations
- NE
norepinephrine
- SNS
sympathetic nervous system
- ST
standard temperature housing (approx. 22°C)
- TT
thermoneutral temperature housing (approx. 30°C)
Footnotes
Authors have no conflicts of interest to declare.
References
- 1.Zitvogel L, Apetoh L, Ghiringhelli F, Andre F, et al. The anticancer immune response: indispensable for therapeutic success? J Clin Invest. 2008;118:1991–2001. doi: 10.1172/JCI35180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Danna EA, Sinha P, Gilbert M, Clements VK, et al. Surgical removal of primary tumor reverses tumor-induced immunosuppression despite the presence of metastatic disease. Cancer Res. 2004;64:2205–11. doi: 10.1158/0008-5472.can-03-2646. [DOI] [PubMed] [Google Scholar]
- 3.Couzin-Frankel J. Breakthrough of the year 2013. Cancer immunotherapy. Science. 2013;342:1432–3. doi: 10.1126/science.342.6165.1432. [DOI] [PubMed] [Google Scholar]
- 4.Gordon CJ. Thermal physiology of laboratory mice: Defining thermoneutrality. J Therm Biol. 2012;37:654–85. [Google Scholar]
- 5.Karp CL. Unstressing intemperate models: how cold stress undermines mouse modeling. J Exp Med. 2012;209:1069–74. doi: 10.1084/jem.20120988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wartha K, Herting F, Hasmann M. Fit-for purpose use of mouse models to improve predictivity of cancer therapeutics evaluation. Pharmacol Ther. 2014;142:351–61. doi: 10.1016/j.pharmthera.2014.01.001. [DOI] [PubMed] [Google Scholar]
- 7.Martin B, Ji S, Maudsley S, Mattson MP. “Control” laboratory rodents are metabolically morbid: why it matters. Proc Natl Acad Sci USA. 2010;107:6127–33. doi: 10.1073/pnas.0912955107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hall JE, Guyton AC. Guyton and Hall Textbook of Medical Physiology. Saunders/Elsevier; 2011. [Google Scholar]
- 9.Cannon B, Nedergaard J. Nonshivering thermogenesis and its adequate measurement in metabolic studies. J Exp Biol. 2011;214:242–53. doi: 10.1242/jeb.050989. [DOI] [PubMed] [Google Scholar]
- 10.Blumberg MS. Body Heat. Harvard University Press; 2002. [Google Scholar]
- 11.Guide for the Care and Use of Laboratory Animals. 8. The National Academies Press; 2011. [PubMed] [Google Scholar]
- 12.Gordon CJ. Temperature regulation in laboratory rodents Cambridge [England]; New York, NY, USA: Cambridge University Press; 1993. [Google Scholar]
- 13.Gaskill BN, Rohr SA, Pajor EA, Lucas JR, et al. Some like it hot: Mouse temperature preferences in laboratory housing. Appl Anim Behav Sci. 2009;116:279–85. [Google Scholar]
- 14.Gaskill BN, Rohr SA, Pajor EA, Lucas JR, et al. Working with what you’ve got: Changes in thermal preference and behavior in mice with or without nesting material. J Therm Biol. 2011;36:193–9. [Google Scholar]
- 15.Kokolus KM, Capitano ML, Lee CT, Eng JW, et al. Baseline tumor growth and immune control in laboratory mice are significantly influenced by subthermoneutral housing temperature. Proc Natl Acad Sci USA. 2013;110:20176–81. doi: 10.1073/pnas.1304291110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gordon CJ, Becker P, Ali JS. Behavioral thermoregulatory responses of single- and group-housed mice. Physiol Behav. 1998;65:255–62. doi: 10.1016/s0031-9384(98)00148-6. [DOI] [PubMed] [Google Scholar]
- 17.Gaskill BN, Gordon CJ, Pajor EA, Lucas JR, et al. Heat or insulation: Behavioral titration of mouse preference for warmth or access to a nest. PLoS One. 2012;7:e32799. doi: 10.1371/journal.pone.0032799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cannon B, Nedergaard J. Thermogenesis challenges the adipostat hypothesis for bodyweight control. Proc Nutr Soc. 2009;68:401–7. doi: 10.1017/S0029665109990255. [DOI] [PubMed] [Google Scholar]
- 19.Serrat MA, King D, Lovejoy CO. Temperature regulates limb length in homeotherms by directly modulating cartilage growth. Proc Natl Acad Sci USA. 2008;105:19348–53. doi: 10.1073/pnas.0803319105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ramanantsoa N, Matrot B, Vardon G, Lajard AM, et al. Impaired ventilatory and thermoregulatory responses to hypoxic stress in newborn phox2b heterozygous knock-out mice. Front Physiol. 2011;2:61. doi: 10.3389/fphys.2011.00061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cummings KJ, Frappell PB. Breath-to-breath hypercapnic response in neonatal rats: temperature dependency of the chemoreflexes and potential implications for breathing stability. Am J Physiol Regul Integr Comp Physiol. 2009;297:R124–34. doi: 10.1152/ajpregu.91011.2008. [DOI] [PubMed] [Google Scholar]
- 22.Lutaif NA, Rocha EM, Veloso LA, Bento LM, et al. Renal contribution to thermolability in rats: role of renal nerves. Nephrol Dial Transplant. 2008;23:3798–805. doi: 10.1093/ndt/gfn368. [DOI] [PubMed] [Google Scholar]
- 23.Virtue S, Even P, Vidal-Puig A. Below thermoneutrality, changes in activity do not drive changes in total daily energy expenditure between groups of mice. Cell Metab. 2012;16:665–71. doi: 10.1016/j.cmet.2012.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ravussin Y, LeDuc CA, Watanabe K, Leibel RL. Effects of ambient temperature on adaptive thermogenesis during maintenance of reduced body weight in mice. Am J Physiol Regul Integr Comp Physiol. 2012;303:R438–48. doi: 10.1152/ajpregu.00092.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lo Martire V, Silvani A, Bastianini S, Berteotti C, et al. Effects of ambient temperature on sleep and cardiovascular regulation in mice: the role of hypocretin/orexin neurons. PLoS One. 2012;7:e47032. doi: 10.1371/journal.pone.0047032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Garami A, Pakai E, Oliveira DL, Steiner AA, et al. Thermoregulatory phenotype of the Trpv1 knockout mouse: thermoeffector dysbalance with hyperkinesis. J Neurosci. 2011;31:1721–33. doi: 10.1523/JNEUROSCI.4671-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mallick HN, Kumar VM. Basal forebrain thermoregulatory mechanism modulates auto-regulated sleep. Front Neurol. 2012;3:102. doi: 10.3389/fneur.2012.00102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Swoap SJ, Li C, Wess J, Parsons AD, et al. Vagal tone dominates autonomic control of mouse heart rate at thermoneutrality. Am J Physiol Heart Circ Physiol. 2008;294:H1581–8. doi: 10.1152/ajpheart.01000.2007. [DOI] [PubMed] [Google Scholar]
- 29.Dacks PA, Rance NE. Effects of estradiol on the thermoneutral zone and core temperature in ovariectomized rats. Endocrinology. 2010;151:1187–93. doi: 10.1210/en.2009-1112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bartke A, Westbrook R, Sun L, Ratajczak M. Links between growth hormone and aging. Endokrynol Pol. 2013;64:46–52. [PMC free article] [PubMed] [Google Scholar]
- 31.Feldmann HM, Golozoubova V, Cannon B, Nedergaard J. UCP1 ablation induces obesity and abolishes diet-induced thermogenesis in mice exempt from thermal stress by living at thermoneutrality. Cell Metabolism. 2009;9:203–9. doi: 10.1016/j.cmet.2008.12.014. [DOI] [PubMed] [Google Scholar]
- 32.Flowers MT, Paton CM, O’Byrne SM, Schiesser K, et al. Metabolic changes in skin caused by Scd1 deficiency: a focus on retinol metabolism. PLoS One. 2011;6:e19734. doi: 10.1371/journal.pone.0019734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Boon MR, van den Berg SA, Wang Y, van den Bossche J, et al. BMP7 activates brown adipose tissue and reduces diet-induced obesity only at subthermoneutrality. PLoS One. 2013;8:e74083. doi: 10.1371/journal.pone.0074083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bartke A, Westbrook R. Metabolic characteristics of long-lived mice. Front Genet. 2012;3:288. doi: 10.3389/fgene.2012.00288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.David JM, Chatziioannou AF, Taschereau R, Wang H, et al. The hidden cost of housing practices: using noninvasive imaging to quantify the metabolic demands of chronic cold stress of laboratory mice. Comp Med. 2013;63:386–91. [PMC free article] [PubMed] [Google Scholar]
- 36.Nagai N, Sakane N, Fujishita A, Fujiwara R, et al. The -3826 A --> G variant of the uncoupling protein-1 gene diminishes thermogenesis during acute cold exposure in healthy children. Obes Res Clin Pract. 2007;1:I–ii. doi: 10.1016/j.orcp.2007.02.001. [DOI] [PubMed] [Google Scholar]
- 37.Fleury C, Neverova M, Collins S, Raimbault S, et al. Uncoupling protein-2: a novel gene linked to obesity and hyperinsulinemia. Nat Genet. 1997;15:269–72. doi: 10.1038/ng0397-269. [DOI] [PubMed] [Google Scholar]
- 38.Kokolus KM, Spangler HM, Povinelli BJ, Farren MR, et al. Stressful presentations: mild cold stress in laboratory mice influences phenotype of dendritic cells in naive and tumor-bearing mice. Front Immunol. 2014;5:23. doi: 10.3389/fimmu.2014.00023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Gabrilovich DI, Ostrand-Rosenberg S, Bronte V. Coordinated regulation of myeloid cells by tumours. Nat Rev Immunol. 2012;12:253–68. doi: 10.1038/nri3175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ostrand-Rosenberg S, Sinha P, Beury DW, Clements VK. Cross-talk between myeloid-derived suppressor cells (MDSC), macrophages, and dendritic cells enhances tumor-induced immune suppression. Semin Cancer Biol. 2012;22:275–81. doi: 10.1016/j.semcancer.2012.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Thaker PH, Han LY, Kamat AA, Arevalo JM, et al. Chronic stress promotes tumor growth and angiogenesis in a mouse model of ovarian carcinoma. Nat Med. 2006;12:939–44. doi: 10.1038/nm1447. [DOI] [PubMed] [Google Scholar]
- 42.Lutgendorf SK, Cole S, Costanzo E, Bradley S, et al. Stress-related mediators stimulate vascular endothelial growth factor secretion by two ovarian cancer cell lines. Clin Cancer Res. 2003;9:4514–21. [PubMed] [Google Scholar]
- 43.Guo K, Ma Q, Wang L, Hu H, et al. Norepinephrine-induced invasion by pancreatic cancer cells is inhibited by propranolol. Oncol Rep. 2009;22:825–30. doi: 10.3892/or_00000505. [DOI] [PubMed] [Google Scholar]
- 44.Cole SW, Sood AK. Molecular pathways: beta-adrenergic signaling in cancer. Clin Cancer Res. 2012;18:1201–6. doi: 10.1158/1078-0432.CCR-11-0641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Magnon C, Hall SJ, Lin J, Xue X, et al. Autonomic nerve development contributes to prostate cancer progression. Science. 2013;341:1236361. doi: 10.1126/science.1236361. [DOI] [PubMed] [Google Scholar]
- 46.Elenkov IJ, Wilder RL, Chrousos GP, Vizi ES. The sympathetic nerve--an integrative interface between two supersystems: the brain and the immune system. Pharmacol Rev. 2000;52:595–638. [PubMed] [Google Scholar]
- 47.Muthu K, Iyer S, He LK, Szilagyi A, et al. Murine hematopoietic stem cells and progenitors express adrenergic receptors. J Neuroimmunol. 2007;186:27–36. doi: 10.1016/j.jneuroim.2007.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Powell ND, Sloan EK, Bailey MT, Arevalo JM, et al. Social stress up-regulates inflammatory gene expression in the leukocyte transcriptome via beta-adrenergic induction of myelopoiesis. Proc Natl Acad Sci USA. 2013;110:16574–9. doi: 10.1073/pnas.1310655110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Jin J, Wang X, Wang Q, Guo X, et al. Chronic psychological stress induces the accumulation of myeloid-derived suppressor cells in mice. PLoS One. 2013;8:e74497. doi: 10.1371/journal.pone.0074497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Cohen MJ, Shankar R, Stevenson J, Fernandez R, et al. Bone marrow norepinephrine mediates development of functionally different macrophages after thermal injury and sepsis. Ann Surg. 2004;240:132–41. doi: 10.1097/01.sla.0000130724.84914.d6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Lucas D, Bruns I, Battista M, Mendez-Ferrer S, et al. Norepinephrine reuptake inhibition promotes mobilization in mice: potential impact to rescue low stem cell yields. Blood. 2012;119:3962–5. doi: 10.1182/blood-2011-07-367102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Katayama Y, Battista M, Kao WM, Hidalgo A, et al. Signals from the sympathetic nervous system regulate hematopoietic stem cell egress from bone marrow. Cell. 2006;124:407–21. doi: 10.1016/j.cell.2005.10.041. [DOI] [PubMed] [Google Scholar]
- 53.Recalde A, Richart A, Guerin C, Cochain C, et al. Sympathetic nervous system regulates bone marrow-derived cell egress through endothelial nitric oxide synthase activation: role in postischemic tissue remodeling. Arterioscler Thromb Vasc Biol. 2012;32:643–53. doi: 10.1161/ATVBAHA.111.244392. [DOI] [PubMed] [Google Scholar]
- 54.Maestroni GJ. Sympathetic nervous system influence on the innate immune response. Ann N Y Acad Sci. 2006;1069:195–207. doi: 10.1196/annals.1351.017. [DOI] [PubMed] [Google Scholar]
- 55.Lutgendorf SK, DeGeest K, Dahmoush L, Farley D, et al. Social isolation is associated with elevated tumor norepinephrine in ovarian carcinoma patients. Brain Behav Immun. 2011;25:250–5. doi: 10.1016/j.bbi.2010.10.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Demir IE, Friess H, Ceyhan GO. Nerve-cancer interactions in the stromal biology of pancreatic cancer. Front Physiol. 2012;3:97. doi: 10.3389/fphys.2012.00097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Brown SW, Meyers RT, Brennan KM, Rumble JM, et al. Catecholamines in a macrophage cell line. J Neuroimmunol. 2003;135:47–55. doi: 10.1016/s0165-5728(02)00435-6. [DOI] [PubMed] [Google Scholar]
- 58.Engler KL, Rudd ML, Ryan JJ, Stewart JK, et al. Autocrine actions of macrophage-derived catecholamines on interleukin-1 beta. J Neuroimmunol. 2005;160:87–91. doi: 10.1016/j.jneuroim.2004.11.005. [DOI] [PubMed] [Google Scholar]
- 59.Flierl MA, Rittirsch D, Nadeau BA, Sarma JV, et al. Upregulation of phagocyte-derived catecholamines augments the acute inflammatory response. PLoS One. 2009;4:e4414. doi: 10.1371/journal.pone.0004414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Nguyen KD, Qiu Y, Cui X, Goh YPS, et al. Alternatively activated macrophages produce catecholamines to sustain adaptive thermogenesis. Nature. 2011;480:104–8. doi: 10.1038/nature10653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Cosentino M, Fietta AM, Ferrari M, Rasini E, et al. Human CD4+CD25+ regulatory T cells selectively express tyrosine hydroxylase and contain endogenous catecholamines subserving an autocrine/paracrine inhibitory functional loop. Blood. 2007;109:632–42. doi: 10.1182/blood-2006-01-028423. [DOI] [PubMed] [Google Scholar]
- 62.Sorriento D, Santulli G, Del Giudice C, Anastasio A, et al. Endothelial cells are able to synthesize and release catecholamines both in vitro and in vivo. Hypertension. 2012;60:129–36. doi: 10.1161/HYPERTENSIONAHA.111.189605. [DOI] [PubMed] [Google Scholar]
- 63.Sirivelu MP, MohanKumar PS, MohanKumar SM. Interleukin-1 beta simultaneously affects the stress and reproductive axes by modulating norepinephrine levels in different brain areas. Life Sci. 2012;91:878–84. doi: 10.1016/j.lfs.2012.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Yu Y, Zhang ZH, Wei SG, Serrats J, et al. Brain perivascular macrophages and the sympathetic response to inflammation in rats after myocardial infarction. Hypertension. 2010;55:652–9. doi: 10.1161/HYPERTENSIONAHA.109.142836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Bardou I, Kaercher RM, Brothers HM, Hopp SC, et al. Age and duration of inflammatory environment differentially affect the neuroimmune response and catecholaminergic neurons in the midbrain and brainstem. Neurobiol Aging. 2014;35:1065–73. doi: 10.1016/j.neurobiolaging.2013.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Guereschi MG, Araujo LP, Maricato JT, Takenaka MC, et al. Beta2-adrenergic receptor signaling in CD4+ Foxp3+ regulatory T cells enhances their suppressive function in a PKA-dependent manner. Eur J Immunol. 2013;43:1001–12. doi: 10.1002/eji.201243005. [DOI] [PubMed] [Google Scholar]
- 67.Vida G, Pena G, Kanashiro A, Thompson-Bonilla Mdel R, et al. beta2-Adrenoreceptors of regulatory lymphocytes are essential for vagal neuromodulation of the innate immune system. Faseb j. 2011;25:4476–85. doi: 10.1096/fj.11-191007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Yang JH, Lee EO, Kim SE, Suh YH, et al. Norepinephrine differentially modulates the innate inflammatory response provoked by amyloid-beta peptide via action at beta-adrenoceptors and activation of cAMP/PKA pathway in human THP-1 macrophages. Exp Neurol. 2012;236:199–206. doi: 10.1016/j.expneurol.2012.05.008. [DOI] [PubMed] [Google Scholar]
- 69.Riether C, Kavelaars A, Wirth T, Pacheco-Lopez G, et al. Stimulation of beta(2)-adrenergic receptors inhibits calcineurin activity in CD4(+) T cells via PKA-AKAP interaction. Brain Behav Immun. 2011;25:59–66. doi: 10.1016/j.bbi.2010.07.248. [DOI] [PubMed] [Google Scholar]
- 70.Takayanagi Y, Osawa S, Ikuma M, Takagaki K, et al. Norepinephrine suppresses IFN-gamma and TNF-alpha production by murine intestinal intraepithelial lymphocytes via the beta(1) adrenoceptor. J Neuroimmunol. 2012;245:66–74. doi: 10.1016/j.jneuroim.2012.02.007. [DOI] [PubMed] [Google Scholar]
- 71.Wang L, Liu H, Chen X, Zhang M, et al. Immune sculpting of norepinephrine on MHC-I, B7-1, IDO and B7-H1 expression and regulation of proliferation and invasion in pancreatic carcinoma cells. PLoS One. 2012;7:e45491. doi: 10.1371/journal.pone.0045491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Speakman JR, Keijer J. Not so hot: Optimal housing temperatures for mice to mimic the thermal environment of humans. Mol Metab. 2012;2:5–9. doi: 10.1016/j.molmet.2012.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Gavrilovic L, Spasojevic N, Dronjak S. Chronic individual housing-induced stress decreased expression of catecholamine biosynthetic enzyme genes and proteins in spleen of adult rats. Neuroimmunomodulation. 2010;17:265–9. doi: 10.1159/000290042. [DOI] [PubMed] [Google Scholar]
- 74.Madden KS, Szpunar MJ, Brown EB. Early impact of social isolation and breast tumor progression in mice. Brain Behav Immun. 2013;30(Suppl):S135–41. doi: 10.1016/j.bbi.2012.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Gibb J, Hayley S, Gandhi R, Poulter MO, et al. Synergistic and additive actions of a psychosocial stressor and endotoxin challenge: Circulating and brain cytokines, plasma corticosterone and behavioral changes in mice. Brain Behav Immun. 2008;22:573–89. doi: 10.1016/j.bbi.2007.12.001. [DOI] [PubMed] [Google Scholar]
- 76.Schuller HM, Al-Wadei HA, Ullah MF, Plummer HK., 3rd Regulation of pancreatic cancer by neuropsychological stress responses: a novel target for intervention. Carcinogenesis. 2012;33:191–6. doi: 10.1093/carcin/bgr251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Thornton LM, Andersen BL, Blakely WP. The pain, depression, and fatigue symptom cluster in advanced breast cancer: covariation with the hypothalamic-pituitary-adrenal axis and the sympathetic nervous system. Health Psychol. 2010;29:333–7. doi: 10.1037/a0018836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Leonard BE. Stress, norepinephrine and depression. J Psychiatry Neurosci. 2001;26(Suppl):S11–6. [PMC free article] [PubMed] [Google Scholar]
- 79.Kulp GA, Herndon DN, Lee JO, Suman OE, et al. Extent and magnitude of catecholamine surge in pediatric burned patients. Shock. 2010;33:369–74. doi: 10.1097/SHK.0b013e3181b92340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Fitzgerald PJ. Elevated norepinephrine may be an etiological factor in a wide range of diseases: age-related macular degeneration, systemic lupus erythematosus, atrial fibrillation, metabolic syndrome. Med Hypotheses. 2013;80:558–63. doi: 10.1016/j.mehy.2013.01.018. [DOI] [PubMed] [Google Scholar]
- 81.Fitzgerald PJ. Beta blockers, norepinephrine, and cancer: an epidemiological viewpoint. Clin Epidemiol. 2012;4:151–6. doi: 10.2147/CLEP.S33695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Demas GE, Chefer V, Talan MI, Nelson RJ. Metabolic costs of mounting an antigen-stimulated immune response in adult and aged C57BL/6J mice. Am J Physiol. 1997;273:R1631–7. doi: 10.1152/ajpregu.1997.273.5.R1631. [DOI] [PubMed] [Google Scholar]
- 83.Lochmiller RL, Deerenberg C. Trade-offs in evolutionary immunology: just what is the cost of immunity? Oikos. 2000;88:87–98. [Google Scholar]
- 84.Downs CJ, Brown JL, Wone B, Donovan ER, et al. Selection for increased mass-independent maximal metabolic rate suppresses innate but not adaptive immune function. Proc Biol Sci. 2013;280:20122636. doi: 10.1098/rspb.2012.2636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Kokolus KM, Hong CC, Repasky EA. Feeling too hot or cold after breast cancer: is it just a nuisance or a potentially important prognostic factor? Int J Hyperthermia. 2010;26:662–80. doi: 10.3109/02656736.2010.507235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Gaskill BN, Gordon CJ, Pajor EA, Lucas JR, et al. Impact of nesting material on mouse body temperature and physiology. Physiol Behav. 2013;110–111:87–95. doi: 10.1016/j.physbeh.2012.12.018. [DOI] [PubMed] [Google Scholar]
- 87.Cannon B, Nedergaard J. Brown adipose tissue: Function and physiological significance. Physiol Rev. 2004;84:277–359. doi: 10.1152/physrev.00015.2003. [DOI] [PubMed] [Google Scholar]
- 88.Uchida K, Shiuchi T, Inada H, Minokoshi Y, et al. Metabolic adaptation of mice in a cool environment. Pflugers Arch. 2010;459:765–74. doi: 10.1007/s00424-010-0795-3. [DOI] [PubMed] [Google Scholar]