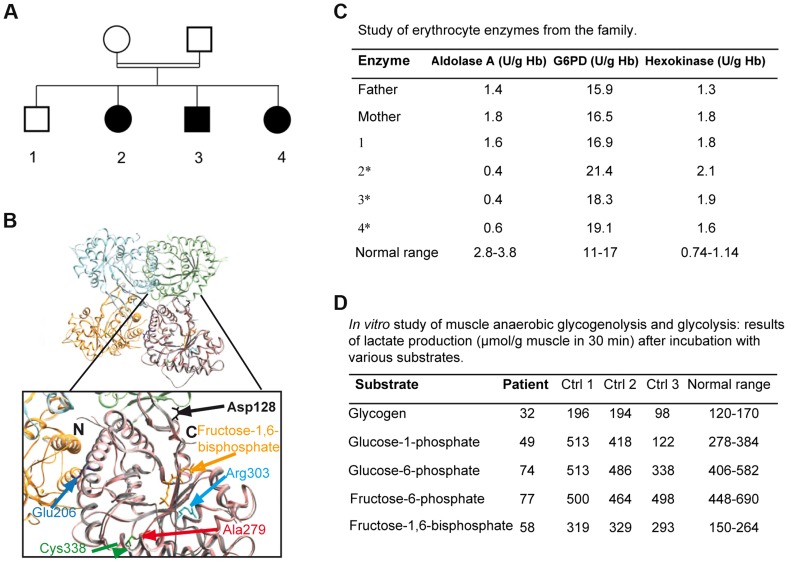
Figure 1. 1A: Family tree showing the 3 affected children.
1B: Crystal structure of human muscle aldolase complexed with fructose 1,6-bisphosphate (isoenzyme A, PDB code 4ALD) superimposed with the tetrameric crystal structure of human brain aldolase (isoenzyme C, PDB code 1XFB), which is similar to the muscle isoenzyme. Chains A, B, C and D of isoenzyme C are shown in orange, light blue, light green and pink, respectively. Monomeric isoenzyme A is shown in grey and is superimposed on chain D of the tetrameric isoenzyme C. Fructose 1,6-bisphosphate co-crystallized with isoenzyme A is shown in yellow. The mutated residue described in this report (red arrow) and the mutated amino acids previously described are highlighted in the magnified structure. The structural and functional consequences of the mutations are described in Table 1. 1C: aldolase A, glucose-6-phosphate dehydrogenase (G6PD) and hexokinase activities in the erythrocytes of the parents, the healthy sibling and the 3 affected patients (*: patients 2, 3, 4). 1D: in vitro muscle study of anaerobic glycogenolysis and glycolysis (only patient 3); results of lactate production (µmol/g muscle in 30 minutes) after incubation with various substrates.