A model of O2 sensing by glomus cells during moderate to severe hypoxia.
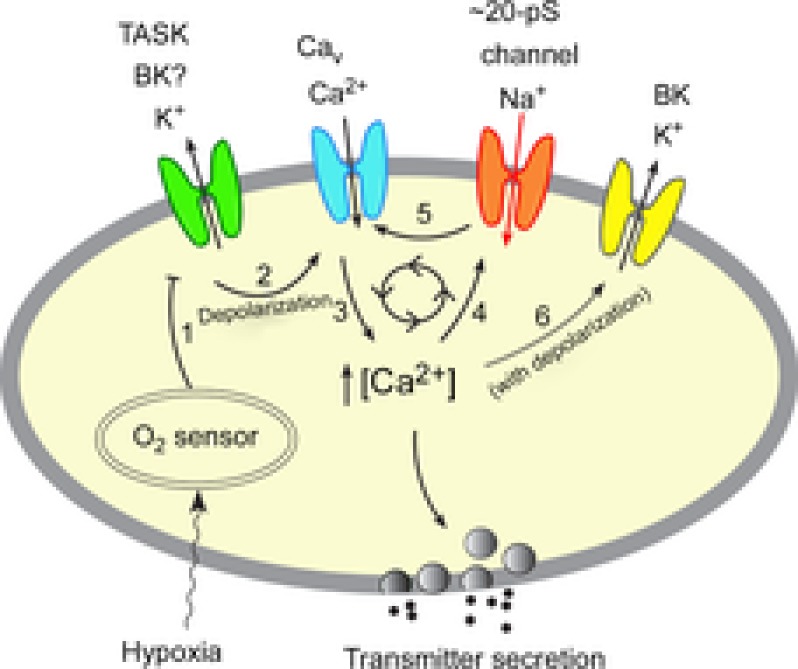
Mild hypoxia (>5% O2) inhibits (step 1) the outward K+ current such as TASK via an O2 sensor. This depolarizes the cell, causing opening of the voltage-gated Ca2+ channels (step 2), influx of Ca2+ and elevation of [Ca2+]i (step 3). The rise in [Ca2+]i causes transmitter secretion. Moderate and severe hypoxia (<5% O2) causes stronger inhibition of the outward K+ current than that produced by mild hypoxia, producing a greater level of depolarization and a higher [Ca2+]i. Rise of [Ca2+]i beyond ∼200 nm from a basal level of ∼100 nm begins to activate the Na+-permeable 20 pS channel (step 4). Na+ influx via the 20 pS channel may initiate a feed-forward mechanism (steps 5→3→4→5) to further increase and maintain the levels of depolarization and [Ca2+]i during moderate to severe hypoxia. Depolarization and the associated increase in [Ca2+]i may activate BK (step 6) and limits the feed-forward mechanism and over-depolarization (see text for further explanation). Thus, our model incorporates the role of both K+- and Na+-permeable channels in the depolarization of glomus cells when hypoxia is moderate to severe.
