FIGURE 2:
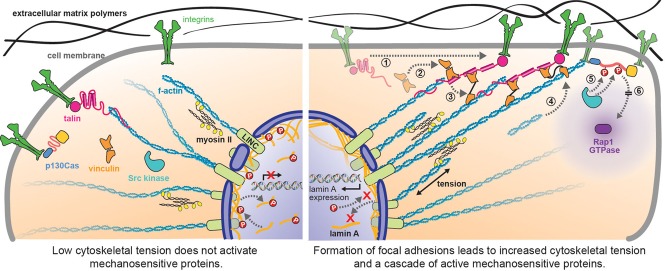
Forces in cells are routed, transmitted, and transduced by mechanically sensitive proteins and have cell-wide implications, influencing biochemical signaling in the cytoplasm and gene expression in the nucleus. Forces generated in the actin cytoskeleton by myosin II cross-bridges are transmitted several micrometers between adhesion proteins in cell membrane and LINC complexes in the nuclear cortex. Tension-dependent unfolding of talin (step 1) reveals substrates for vinculin binding (step 2), which in turn recruits additional actin filaments (steps 3 and 4) as part of focal adhesion development (del Rio et al., 2009; Ciobanasu et al., 2014; Yao et al., 2014). Tension-dependent unfolding of p130Cas reveals phosphorylation sites for Src kinase as part of integrin signaling and ultimately generates the active form of a diffusible GTPase Rap1(steps 5 and 6; Sawada et al., 2006). Concomitantly, tension in the actin cytoskeleton is transmitted through LINC complexes to the nuclear cortex. Lamin A, an intermediate filament of the nuclear cortex, mechanically couples the nuclear cortex to LINC complexes and therefore the cytoplasmic cytoskeleton; it affects DNA transcription of the gene for lamin A and the transcription of stress fiber genes (Swift et al., 2013). Increased cytoskeletal tension on LINC complexes correlates with decreasing phosphorylation of lamin A, decreasing turnover of lamin A in the nuclear cortex, increasing stiffness of the nuclear cortex, and ultimately, through the retnonic acid pathway, increasinglevels of lamin A. Note that many intermediate proteins are not shown, for simplicity.
