Abstract
OBJECTIVE
To evaluate associations between baseline characteristics, nerve-sparing (NS) status and return of continence, as a relationship may exist between return to continence and preservation of the neurovascular bundles for potency during radical prostatectomy (RP).
PATIENTS AND METHODS
The study included 592 consecutive robotic RPs completed between 2002 and 2007.
All data were entered prospectively into an electronic database.
Continence data (defined as zero pads) was collected using self-administered validated questionnaires.
Baseline characteristics (age, International Index of Erectile Function [IIEF-5] score, American Urological Association symptom score, body mass index [BMI], clinical T-stage, Gleason score, and prostate-specific antigen level), NS status and learning curve were retrospectively evaluated for association with overall continence at 1, 3 and 12 months after RP using univariate and multivariable methods.
Any patient taking preoperative phosphodiesterase inhibitors was excluded from the postoperative analysis.
RESULTS
Complete data were available for 537 of 592 patients (91%).
Continence rates at 12 months after RP were 89.2%, 88.9% and 84.8% for bilateral NS, unilateral NS and non-NS respectively (P = 0.56).
In multivariable analysis age, IIEF-5 score and BMI were significant independent predictors of continence.
Cavernosal NS status did not significantly affect continence after adjusting for other co-variables.
CONCLUSION
After careful multivariable analysis of baseline characteristics age, IIEF-5 score and BMI affected continence in a statistically significant fashion. This suggests that baseline factors and not the physical preservation of the cavernosal nerves predict overall return to continence.
Keywords: urological surgery procedures, prostatectomy, treatment outcomes, urinary incontinence, minimally invasive surgical procedures, robotic
INTRODUCTION
For nearly three decades improved continence after radical prostatectomy (RP) has been linked with sparing of the cavernosal nerves [1,2]. The relationship between potency and continence has been variably attributed to a meticulous dissection of the neurovascular bundles (NVBs), apex and continence mechanisms. Several authors have reported that men undergoing bilateral nerve-sparing (BNS) have quicker and better recovery of continence then men undergoing unilateral NS (UNS) or non-NS (NNS) surgery. This suggests that the nerves for continence run with the NVBs and partial or complete resection of one or both nerves may be associated with increased time to recovery of continence and/or overall incontinence. While much has been written about meticulous technique while preserving the cavernosal nerves, there is no uniform definition of what constitutes a NNS procedure. Unilateral or bilateral NNS is not equivalent to unilateral or bilateral wide excision of the NVB. Dr Walsh states in Campbell’s Urology [3] that wide excision means removal of the NVB ‘from the apex laterally to the tip of the seminal vesicle’. Thus, it is difficult to differentiate between men with preoperative sexual dysfunction who undergo a RP with no definitive effort to preserve the cavernosal nerves, and those who have a bilateral wide excision of the NVBs due to high volume or more aggressive cancer.
In the present study, we evaluated baseline characteristics such as: age, International Index of Erectile Function (IIEF-5) score, body mass index (BMI), AUA symptom and bother score (AUAss), clinical T-stage, Gleason score, and PSA level, as well as NS status on the time to recovery of zero-pad status and overall zero-pad continence (ZPC).
PATIENTS AND METHODS
Between June 2002 and December 2007, 592 consecutive patients underwent robot-assisted RP by one surgeon (T. A.). All patient data including baseline characteristic such as age, height, weight, clinical T-stage and Gleason score, PSA level, IIEF-5 score (Table 1), and pertinent medical history was collected and entered prospectively into a dedicated electronic database at the time of RP. Urinary and sexual outcomes were obtained by validated self-administered questionnaires at 3-month intervals, including selected questions from the Expanded Prostate Cancer Index Composite(EPIC-26) questionnaire, the seven-item AUAss and the IIEF-5. Continence was defined as the use of no urinary pads. Men using pads (any size or security) were not considered continent. Also, to help corroborate the questionnaires given every 3 months, patients were also given pre-printed postcards and instructed to mail in self-reported status the week that they stopped using pads. NS status was prospectively recorded and precisely defined. BNS included all patients undergoing bilateral complete or partial nerve preservation. UNS only included patients undergoing wide excision of one nerve; NNS included only men undergoing bilateral wide excision of both NVBs. Institutional Review Board approval was granted for the study. A non-clinical research associate collected all follow-up information.
TABLE 1.
The patients’ characteristics
| Variable | NNS
|
UNS
|
BNS
|
F-test | P | |||
|---|---|---|---|---|---|---|---|---|
| N (%) | Mean (SE) | N (%) | Mean (SE) | N (%) | Mean (SE) | |||
| Age, years | 37 | 66.54 (1.02) | 143 | 62.69 (0.63) | 357 | 60.02 (0.37) | 22.49 | <0.001 |
| PSA level, ng/mL | 37 | 13.25 (2.98) | 143 | 7.04 (0.39) | 357 | 6.07 (0.21) | 22.49 | <0.001 |
| AUAss | 37 | 10.41 (1.23) | 143 | 9.21 (0.62) | 357 | 8.35 (0.37) | 1.87 | 0.156 |
| IIEF-5* score | 36 | 8.67 (1.25) | 143 | 17.69 (0.64) | 352 | 20.89 (0.31) | 62.26 | <0.001 |
| Prostate weight, g | 37 | 54.53 (3.93) | 142 | 47.93 (1.27) | 350 | 52.11 (1.17) | 2.64 | 0.073 |
| BMI, kg/m2 | 36 | 27.79 (0.61) | 142 | 27.12 (0.30) | 338 | 26.63 (0.18) | 2.49 | 0.085 |
| c-Stage: | ||||||||
| I | 17 (46) | 53 (37) | 282 (79) | Pearson | <0.001 | |||
| II–III | 20 (54) | 90 (63) | 75 (21) | Chi-square | ||||
excludes 14 patients taking preoperative PDE-5 inhibitors.
Our surgical technique has been previously described, which includes a running Van Velthovenurethrovesical anastomosis [4]. Over the course of this experience, we had one major technical alteration relating to potency where the vascular pedicle and dissection of the NVBs was performed in an athermal fashion. In cases #1–15 the vascular pedicle was divided with monopolar cautery; in cases #16–125 the vascular pedicle was divided with bipolar cautery and scissors [5] and from case #126–592 we used a cautery-free technique using either bulldog clamps or suture ligature [6].
In all cases we used an antegrade inter-fascial approach to NS. We defined UNS as preservation of one NVB using a standard inter-fascial technique and wide excision of the entire NVB on the contralateral side. This information was recorded prospectively at the time of surgery by the operating surgeon. Wide excision includes all tissue from the midline of the rectum from the bladder neck to the urogenital diaphragm. Briefly, wide excision of the NVB was performed by transecting the vascular pedicle ≈1 cm from the base of the prostate. After dissecting through the peri-rectal fat, the anterior surface of the rectum is identified by visualizing the longitudinal muscle fibres of the rectum. The dissection is carried laterally to the muscles of the levatorani, which results in complete excision of the entire NVB down to the apex. All partial excisions of the NVB were categorized as BNS. To physically verify confidence regarding unilateral wide excision and unilateral NNS status we randomly selected 17 cases undergoing unilateral wide excision for pathological review. Our uropathologist performed a ‘blinded’ review. We measured the maximum distance (mm) from the prostatic capsule to the specimen edge (extraprostatic tissue) posterio-laterally. In this fashion, each patient served as its own control as there was wide excision on one side and NS (partial or total) on the other. The bladder neck was dissected from the prostate in standard fashion and no reconstructive measures such as a Rocco suture or anterior suspension were used.
All data was analysed retrospectively. The primary clinical outcome was use of no urinary pads at 1, 3 and 12 months after RP. Groups defined by NS procedure were compared for baseline co-variables using one-way ANOVA. In all, 14 patients who used phosphodiesterase inhibitors (Viagra or Cialis)before RP and seven with incomplete IIEF-5 assessments were excluded from the analysis. For univariate analysis of continence rates Cochran’s test for linear trend was used. Logistic regression analysis was used to test for differences in continence associated with NS procedure (UNS and BNS each characterized as a dichotomous variable relative to NNS) after adjusting for baseline characteristics, including age, AUAss, IIEF-5 score, BMI, prostate weight, clinical stage, and learning curve (consecutive case number) that could independently affect the return to continence. A P < 0.05was considered to indicate statistical significance.
RESULTS
In all, 537 of 592 (91%) patients had follow-up data for continence (Table 1). For the purposes of this study, a patient was followed until ZPC was achieved. If a patient did not reach zero-pads, he was followed indefinitely or until lost to follow-up. Patients were analysed for wide excision as follows: UNS (143 patients), NNS (37) and BNS (357).
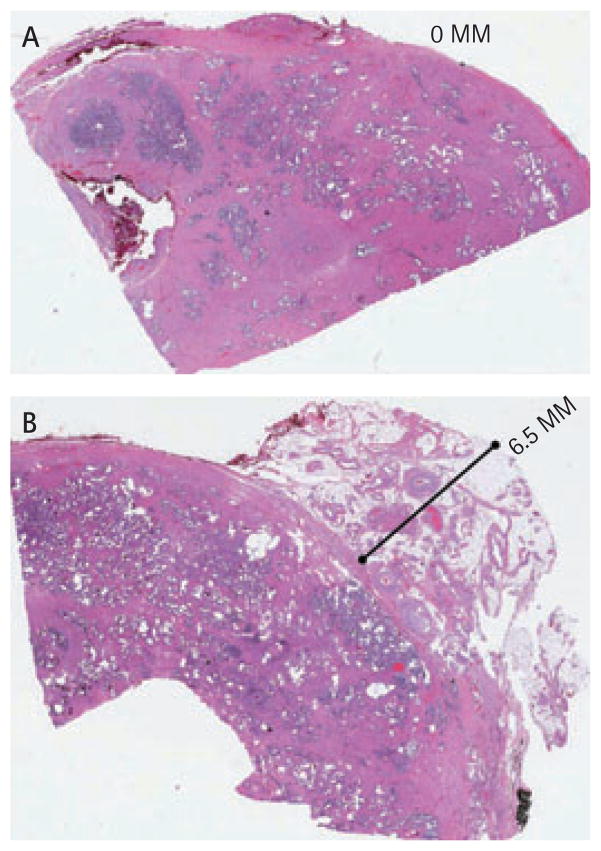
In all, 17 patients undergoing unilateral wide excision had the following findings. On the side of NS the mean (range) extraprostatic tissue was 2.03 (0–3.5) mm; for the side with wide excision the mean distance of extraprostatic tissue was 7.97 (4.5–13.0) mm, which was statistically significant (P < 0.001). Figure 1 shows examples of NS and wide excisions.
FIG. 1.
Cross sections of the prostate showing the amount of extraprostatic tissue with the specimen for (A) nerve sparing and (B) non nerve sparing (wide excision) robotic prostatectomies.
The overall ZPC rate at 12 months after RP for all patients was 88.8%. Among patients who underwent BNS, UNS and NNS, continence rates at 12 months were 89.2%, 88.9%, and 84.8%, respectively (chi-square test for trend, P = 0.563). In all, 56 patients failed to reach continence at ≤12 months: 36 BNS, 15 UNS and five NNS. Overall, NS did not show a clear statistically significant effect on continence at 1, 3 or 12 months follow-up (P = 0.39, P = 0.66 and P = 0.56 for 1, 3 and 12 months respectively; Table 2).
TABLE 2.
Continence rates by NS status
| NNS, n/N (%) | UNS, n/N (%) | BNS, n/N (%) | P* | |
|---|---|---|---|---|
| Continence at 1 month | 10/37 (27.0) | 43/143 (30.1) | 117/357 (32.8) | 0.390 |
| Continence at 3 months | 23/37 (62.2) | 91/141 (64.5) | 233/355 (65.6) | 0.661 |
| Continence at 12 months | 28/33 (84.8) | 120/135 (88.9) | 296/332 (89.2) | 0.563 |
Chi-square test for trend.
Analysis of baseline data showed statistically significant differences between the three NS groups (Table 1). The mean age, PSA level, IIEF-5 score, and clinical stage were significantly different between patients that underwent BNS, UNS and NNS. In general, the NNS patients were significantly older, had significantly higher PSA values and clinical stage, and significantly lower IIEF-5 scores than either UNS or BNS patients. Associations between baseline co-variables and ZPC were investigated using a logistic regression model. Age, IIEF-5 score and BMI were significant predictors of continence at 1, 3 and 12 months after RP (Table 3).
TABLE 3.
Relative odds for ZPC associated with baseline covariables
| Continence at 1 month
|
Continence at 3 months
|
Continence at 12 months
|
||||
|---|---|---|---|---|---|---|
| OR (95% CI) | P | OR (95% CI) | P | OR (95% CI) | P | |
| Age (continuous) | 0.95 (0.92–0.98) | 0.001 | 0.94 (0.91–0.97) | <0.001 | 0.95 (0.91–1.00) | 0.064 |
| IIEF-5 score (≤16, 17–22, ≥23) | 1.48 (1.13–1.94) | 0.005 | 1.39 (1.07–1.79) | 0.013 | 1.70 (1.14–2.55) | 0.010 |
| Prostate weight (≤58, >58 g) | 0.74 (0.46–1.20) | 0.227 | 0.65 (0.42–1.02) | 0.059 | 0.54 (0.28–1.03) | 0.061 |
| BMI (≤30, >30 kg/m2) | 0.67 (0.38–1.19) | 0.170 | 0.54 (0.33–0.92) | 0.023 | 0.26 (0.13–0.51) | <0.001 |
| Learning curve, patient no. (#1–125, #126–250, #251–450, #451–592) | 0.72 (0.59–0.87) | 0.009 | 0.71 (0.59–0.86) | <0.001 | 1.04 (0.78–1.40) | 0.775 |
We examined NS in a logistic regression model before and after adjusting for all co-variables that were independently associated with continence (Table 4). While univariate analyses showed NS to be associated with a non-significant increase in the relative odds for continence, NS was not significantly associated with improvement in continence at 1, 3 or 12 months after adjusting for baseline co-variables. Adjusted odds ratios(ORs) were <1, suggesting that the small improvements in unadjusted continence rates with NS can be explained by other prognostic factors. The CIs include 1 and are in fact similar to the 95% CIs for the unadjusted ORs supporting a lack of association between NS and ZPC.
TABLE 4.
Relative odds for ZPC associated with NS
| Unadjusted OR (95% CI) | Adjusted OR* (95% CI) | |
|---|---|---|
| Continence at 1 month | ||
| UNS | 1.16 (0.52–2.61) | 0.73 (0.30–1.77) |
| BNS | 1.32 (0.62–2.81) | 0.74 (0.31–1.75) |
| Continence at 3 months | ||
| UNS | 1.11 (0.52–2.34) | 0.79 (0.35–1.79) |
| BNS | 1.16 (0.58–2.34) | 0.68 (0.31–1.52) |
| Continence at 12 months | ||
| UNS | 1.43 (0.48–4.26) | 0.65 (0.19–2.18) |
| BNS | 1.47 (0.53–4.04) | 0.45 (0.14–1.47) |
Adjusted for age, IIEF-5 score, prostate weight, BMI, and surgeon learning curve. AUAss, PSA level and clinical stage were not independently associated with continence in multivariable analysis and were thus excluded from the model.
DISCUSSION
A confounding problem with many previous studies is that it is unclear as to whether NS meant a definitive effort to preserve sexual function vs the definitive attempt to widely excise one or both nerves. In the present study, we attempted to more precisely define NS status. We only included wide excision of the NVB in UNS. Additionally, we performed a ‘blinded’ pathological review showing a mean (range)of 8 (4.5–13) mm of extraprostatic tissue with wide excision vs 2 (0–3.5) mm of tissue when performing nerve preservation (P < 0.001). Lastly, widely excising one or both nerves was prospectively entered in to an electronic database at the time of RP by the operating surgeon.
As has been reported previously there was a trend in improved rates of continence with univariate analysis of NS. However, after multivariate analysis adjusting for baseline patient characteristics there was no significant association between NS and time to continence at 1, 3, and 12 months after RP. Age, IIEF-5 score and BMI were significant predictors in the present study. This is similar to recent findings by Shikanov et al. [7]. In their study of robotic RPs in elderly patients, they reported that in univariate analysis, UNS or non-NS was significantly associated with lower odds of achieving continence, while there was no significant association between NS and continence with multivariate analysis. Kundu et al. [8], in their series of > 3000 open RPs, did not find a correlation between NS and continence, reporting only that age was significantly associated with continence. For robot-assisted RPs, most data associated with continence have focused on differences in technique that have been associated with early return to continence [9–11]. Mottrie et al. [12]. reported a non-significant relationship between NS and early return to continence in robotic cases, as did Salomon et al. [13] in a review comparing laparoscopic and open RPs.
Two open RP series by Nandipati et al. [14] and Burkhard et al. [15] have reported important findings. Both groups (156 and 536 patients, respectively) performed multivariate analysis. Nandipati et al. [14] included age, PSA level and Gleason score and found a correlation with continence and NS status at 1 year. Similarly, Burkhard et al. [15] also linked continence to NS status in multivariate analysis, but neither included IIEF-5 scores or BMI in their univariate or multivariate analysis.
If a relationship truly exists between NS status and time to continence and or overall continence this suggests that some component of nerves specifically dealing with urinary continence anatomically run with the cavernosal nerves. Two contradictory studies attempted to stimulate the NVB and measure urethral closing pressures. One study reported increased urethral sphincter pressures [16], the other did not [17]. Further in 2009, Tzou et al. [18], reported that when NS surgery resulted in potency, continence did not respond as expected if the nerves run together. In the present study, when evaluating continence in patients undergoing unilateral or bilateral wide excision one would expect an obvious decline in continence, which was not seen.
The present study may suffer from patient selection bias due to the tertiary nature of the surgeon’s practice. These patients may also be more motivated to do Kegel exercises or other bladder training to achieve continence. Finally, the number of patients in the NNS group is small (37), thus limiting power to detect small differences in continence rates between groups. Nevertheless, that the small trend of improved continence rates with UNS and BNS in univariate analyses disappeared after adjustment for baseline co-variables suggests that NS does not affect return to continence after robotic RP.
In conclusion, using a strict definition of NS and urinary continence, and after univariate and multivariate analysis, only age, IIEF-5 score and BMI affected continence in a statistically significant fashion. These results suggest that, anatomically speaking, the cavernosal nerves are distinct from the nerves and muscles controlling continence.
What’s known on the subject? and What does the study add?
Continence after radical prostatectomy (RP) has been linked to surgical techniques including careful dissection of the neurovascular bundles, bladder neck preservation, sparing of the puboprostatic ligaments and reconstruction of the posterior urethral plate or total reconstruction of the vesico-urethral junction. Several authors have reported that men undergoing bilateral nerve-sparing have quicker and better recovery of continence than men undergoing partial or non-nerve-sparing procedures. Others have reported that preoperative variables have a greater effect than technique on postoperative return to continence.
We examine the association between baseline characteristics (age, International Index of Erectile Function [IIEF-5] score, American Urological Association symptom score, body mass index [BMI], clinical T stage, Gleason score, and prostate-specific antigen level), nerve-sparing status, learning curve and overall continence at 1, 3 and 12 months after robotic RP. In addition, nerve-sparing status was physically verified by comparing the amount of extraprostatic tissue seen on the wide excision side and nerve-sparing side for unilateral nerve-sparing procedures. After multivariate analysis, age, IIEF-5 and BMI were found to affect continence in a statistically significant fashion, while nerve-sparing status did not significantly affect continence.
Abbreviations
- RP
radical prostatectomy
- NVB
neurovascular bundle
- IIEF-5
International Index of Erectile Function
- AUAss
AUA BPH Symptom Score Index
- ZPC
zero-pad continence
- BMI
body mass index
- (B)(U)(N)NS
(bilateral) (unilateral) (non)nerve-sparing
- OR
odds ratio
Footnotes
CONFLICT OF INTEREST
None declared.
References
- 1.Stanford JL, Feng ZD, Hamilton AS, et al. Urinary and sexual function after radical prostatectomy for clinically localized prostate cancer: the Prostate Cancer Outcomes Study. JAMA. 2000;283:354–60. doi: 10.1001/jama.283.3.354. [DOI] [PubMed] [Google Scholar]
- 2.Menon M, Tewari A Vattikuti Institute Prostatectomy Team. Robotic radical prostatectomy and the Vattikuti Urology Institute technique: an interim analysis of results and technical points. Urology. 2003;61 (Suppl 1):15–20. doi: 10.1016/s0090-4295(03)00116-x. [DOI] [PubMed] [Google Scholar]
- 3.Walsh PC. Anatomic radical retropubic prostatectomy. In: Walsh PC, Retik AB, Vaughan ED Jr, Wein AJ, editors. Campbell’s Urology. 7. Philadelphia: WB Saunders Inc; 1997. [Google Scholar]
- 4.Matsunaga GS, Ahlering TE, Skarecky DW. Update on robotic laparoscopic radical prostatectomy. Scientific World Journal. 2006;6:2542–52. doi: 10.1100/tsw.2006.394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ahlering TE, Eichel L, Skarecky D. Evaluation of long-term thermal injury using cautery during nerve sparing robotic prostatectomy. Urology. 2008;72:1371–4. doi: 10.1016/j.urology.2007.11.101. [DOI] [PubMed] [Google Scholar]
- 6.Ahlering TE, Eichel L, Chou D, Skarecky DW. Feasibility study for robotic radical prostatectomy cautery-free neurovascular bundle preservation. Urology. 2005;65:994–7. doi: 10.1016/j.urology.2004.11.023. [DOI] [PubMed] [Google Scholar]
- 7.Shikanov S, Desai V, Razmaria A, Zagaja GP, Shalhav AL. Robotic radical prostatectomy for elderly patients: probability of achieving continence and potency 1 year after surgery. J Urol. 2010;183:1803–7. doi: 10.1016/j.juro.2010.01.016. [DOI] [PubMed] [Google Scholar]
- 8.Kundu SD, Roehl KA, Eggener SE, Antenor JAV, Han M, Catalona WJ. Potency, continence and complications in 3,477 consecutive radical retropubic prostatectomies. J Urol. 2004;172:2227–31. doi: 10.1097/01.ju.0000145222.94455.73. [DOI] [PubMed] [Google Scholar]
- 9.Stein RJ. The case for posterior musculofascial plate reconstruction in robotic prostatectomy. Urology. 2009;74:489–91. doi: 10.1016/j.urology.2008.08.521. [DOI] [PubMed] [Google Scholar]
- 10.Tan GY, Jhaveri JK, Tewari AK. Anatomic restoration technique: a biomechanics-based approach for early continence recovery after minimally invasive radical prostatectomy. Urology. 2009;74:492–6. doi: 10.1016/j.urology.2009.02.005. [DOI] [PubMed] [Google Scholar]
- 11.Freire MP, Weinberg AC, Lei Y, et al. Anatomic bladder neck preservation during robotic-assisted laparoscopic radical prostatectomy: description of technique and outcomes. Eur Urol. 2009;56:972–80. doi: 10.1016/j.eururo.2009.09.017. [DOI] [PubMed] [Google Scholar]
- 12.Mottrie A, Van Migem P, De Naeyer G, Schatteman P, Carpentier P, Fonteyne E. Robot-assisted laparoscopic radical prostatectomy: oncologic and functional results of 184 cases. Eur Urol. 2007;52:746–50. doi: 10.1016/j.eururo.2007.02.029. [DOI] [PubMed] [Google Scholar]
- 13.Salomon L, Sèbe P, De la Taille A, et al. Open versus laparoscopic radical prostatectomy: Part II. BJU Int. 2004;94:244–50. doi: 10.1111/j.1464-410X.2004.04951.x. [DOI] [PubMed] [Google Scholar]
- 14.Nandipati KC, Raina R, Agarwal A, Zippe CD. Nerve-sparing surgery significantly affects long-term continence after radical prostatectomy. Urology. 2007;70:1127–30. doi: 10.1016/j.urology.2007.07.042. [DOI] [PubMed] [Google Scholar]
- 15.Burkhard FC, Kessler TM, Fleishmann A, Thalmann GN, Schumaccher M, Studer UE. Nerve sparing open radical retropubic prostatectomy – does it have an impact on urinary continence. J Urol. 2006;176:189–95. doi: 10.1016/S0022-5347(06)00574-X. [DOI] [PubMed] [Google Scholar]
- 16.Nelson CP, Montie JE, McGuire EJ, Wedemeyer G, Wei JT. Intraoperative nerve stimulation with measurement of urethral sphincter pressure changes during radical retropubic prostatectomy: a feasibility study. J Urol. 2003;169:2225–8. doi: 10.1097/01.ju.0000058213.15524.90. [DOI] [PubMed] [Google Scholar]
- 17.Michl UHG, Lange D, Graefen M, Huland H. Re: Intraoperative nerve stimulation with measurement of urethral sphincter pressure changes during radical retropubic prostatectomy: a feasibility study. J Urol. 2004;171:359. doi: 10.1097/01.ju.0000102220.88548.77. [DOI] [PubMed] [Google Scholar]
- 18.Tzou DT, Dalkin BL, Christopher BA, Cui HY. The failure of a nerve sparing template to improve urinary continence after radical prostatectomy: attention to study design. Urol Oncol. 2009;27:358–62. doi: 10.1016/j.urolonc.2008.01.013. [DOI] [PubMed] [Google Scholar]