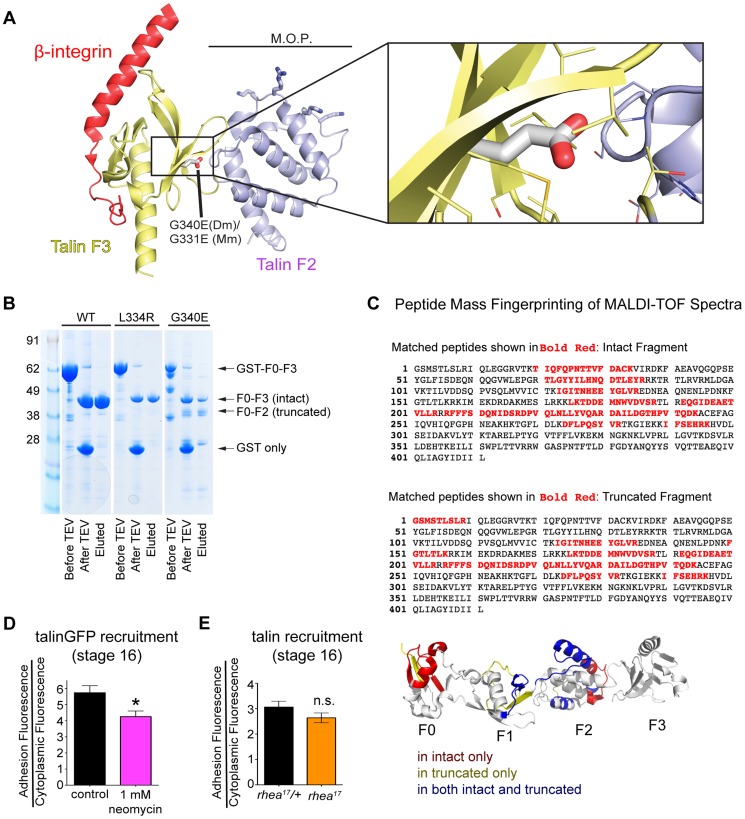
Figure 6. G340 maintains an intermolecular interaction between F2 and F3 that couples their activity.
(a) The conserved role of G340 (G331 in mammalian talin2, shown here) is to stabilize the domain orientation of F2 and F3 (a), which work together to induce integrin activation and stabilize that talin head at the plasma membrane. Modelling based on known structures (see [15]) of mouse talin2 and the integrin cytoplasmic tail suggests that the G340E mutation would disrupt the tight apposition of F2 and F3, thus allowing them to behave as independent modules. (b) In vitro expression of WT (left lane), L334R (middle lane), and G340E (right lane) constructs reveals proteolytic sensitivity of G340E compared to WT and L334R. (c) MALDI-TOF mass spectrometry and peptide mass fingerprinting were used to identify that the sequence of the truncated fragment of the talin head observed in (b) corresponded to the F0-F2 domains indicating F3 was often cleaved in the G340E mutant. (d–e) The recruitment of talin was measured in neomycin treated embryos (d) and in rhea17 embryos (e). Talin recruitment was significantly reduced in neomycin-treated compared to controls (*p<0.05). In contrast there was no such reduction in the rhea17 embryos suggesting the G340E mutant talin protein interacts with the membrane as well as the WT protein.