Abstract
Photoacoustic microscopy (PAM) is capable of mapping microvasculature networks in biological tissue and has demonstrated great potential for biomedical applications. However, the clinical application of the PAM system is limited due to the use of bulky and expensive pulsed laser sources. In this paper, a low-cost optical-resolution PAM system with a pulsed laser diode excitation has been introduced. The lateral resolution of this PAM system was estimated to be 7 µm by imaging a carbon fiber. The phantoms made of polyethylene tubes filled with blood and a mouse ear were imaged to demonstrate the feasibility of this PAM system for imaging biological tissues.
OCIS codes: (110.5120) Photoacoustic imaging, (140.2020) Diode lasers, (170.3880) Medical and biological imaging
1. Introduction
Photoacoustic imaging is a hybrid imaging modality based on optical illumination and ultrasound detection [1–3]. A short-pulsed laser beam is employed for tissue irradiation and an ultrasound transducer is used to receive the photoacoustic signals generated by the tissue absorption of the light [4–6]. Photoacoustic microscopy, especially optical-resolution photoacoustic microscopy (OR-PAM), is capable of mapping microvasculature networks in biological tissue and resolving blood vessels with much higher spatial resolution than conventional photoacoustic imaging with ultrasound array transducers [7–21]. OR-PAM is promising for cancer screening and diagnosis. Xie at al. studied the feasibility of PAM to differentiate malignant bladder tissue from benign one [22]. In our previous study [23], an OR-PAM system using nanosecond pulsed Ti:Sapphire laser source was developed and applied to ovarian cancer detection. A specificity of 81.3% and a sensitivity of 88.2% were achieved in classifying normal and malignant ovarian tissue. However, the excitation source widely used for photoacoustic imaging is bulky and expensive, such as Q-switched Nd:YAG laser, Ti:Sapphire laser, dye laser, and optical parametric oscillator (OPO), etc. Those excitation sources could provide a pulse width of several to tens of nanoseconds and a pulse energy of several to tens of millijoules, but have limitations due to the high cost, bulky size, low pulse repetition rate, and cooling requirement. These factors significantly limit the PAM clinical applications. An inexpensive and compact laser diode could be an effective excitation source for photoacoustic imaging [24–28]. A PAM system with a laser diode excitation was reported and carbon fibers were imaged to test the system [29]. In this paper, a low-cost OR-PAM system with a pulsed laser diode excitation was developed. We demonstrate a laser-diode-based OR-PAM for biological tissue imaging. This study moves one step forward for translating the PAM technique from bench to bedside.
2. Methods and materials
The OR-PAM system configuration is shown in Fig. 1. A pulsed laser diode (Laser Components, 905D4S16S) with a wavelength of 905 nm and output peak power of 130 W was used as an excitation source. The laser diode beam profile is shown in Fig. 2. The pulse repetition rate is 1 KHz, and the pulse width is 124 ns. The laser beam was collimated using a collimation tube (Thorlabs, LT230P-B) consisting of compound lenses, and then focused onto the imaged sample by using a 60X microscope objective lens with a numerical aperture (NA) of 0.7. A function generator was used to provide a TTL (Transistor-Transistor Logic) signal to the driver (Laser Components, LSP-40) of the laser diode, and a synchronized TTL signal was sent to the data acquisition (DAQ) board. Ultrasound (US) gel was used to couple the photoacoustic signal to a single element transducer (Echo, BI933) with a center frequency of 3.5 MHz. The acquired photoacoustic signal was first amplified by a 28 dB preamplifier (Mini-Circuits, ZFL-500LN) and followed by Panametrics receiver with an amplification gain of 59 dB, and then sampled by the DAQ PC. A 3D scanning motor was used to scan the transducer together with the imaged sample to obtain PAM images, and the distance between the microscope objective lens and the imaged sample adjusted to achieve the optimal lateral resolution.
Fig. 1.
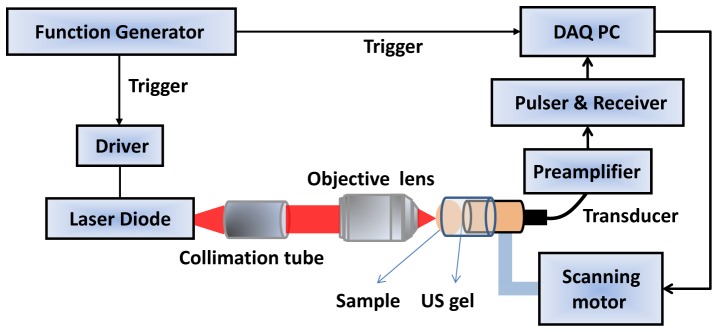
Configuration of the laser-diode PAM system.
Fig. 2.
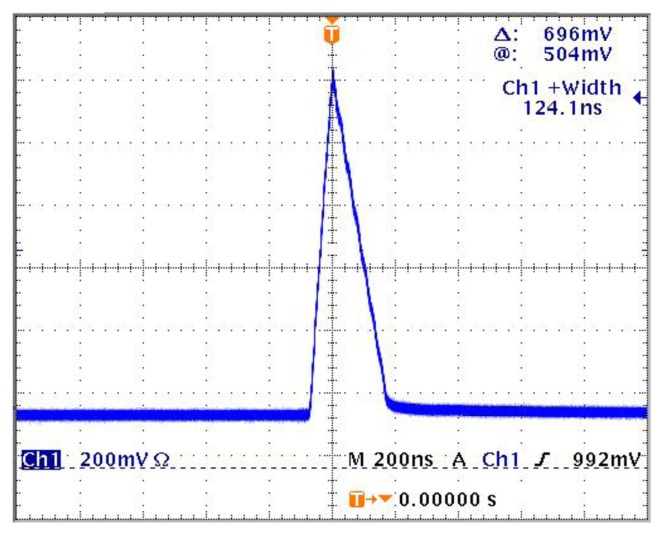
The laser diode beam profile; the pulse width is 124 ns.
3. Results and discussions
The lateral resolution of the laser-diode PAM system was tested by imaging a 7 µm diameter carbon fiber. Figure 3(a) shows the PAM maximum amplitude projection (MAP) image, and Fig. 3(b) shows normalized cross-sectional profile of the carbon fiber along the dotted line shown in Fig. 3(a). The peak-to-peak signal-to-noise ratio (SNR) of photoacoustic signal is 15.7dB. The full width at half maximum (FWHM) was estimated to be 14 µm. The subtraction of the carbon fiber diameter was used to estimate the lateral resolution of the system [10,30]. Therefore, the lateral resolution of the PAM system is ~7 µm.
Fig. 3.
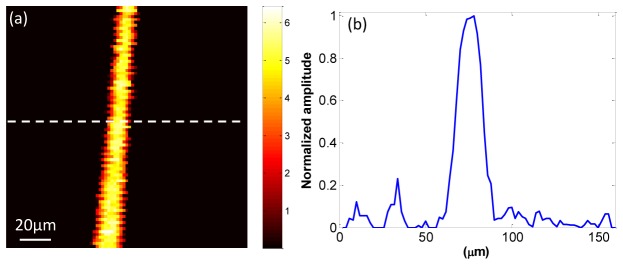
(a) PAM image of a 7 µm carbon fiber; (b) normalized cross-sectional profile of the carbon fiber along the dotted line shown in (a).
The performance of the laser-diode PAM system was tested by imaging two polyethylene tubes filled with the rat blood that mimic the blood vessels. Figure 4(a) shows the photograph of the blood tubes. The left tube has an inner diameter of 0.38 mm and an outer diameter of 1.09 mm; the right tube has an inner diameter of 0.58 mm and an outer diameter of 0.97 mm. Figure 4(b) shows the corresponding PAM MAP image of the two blood-filled tubes. The image clearly displays the two different-diameter tubes with optical absorption contrast. The peak-to-peak SNR of photoacoustic signal is 12.7 dB. The averaged normalized cross-sectional profile of the blood tubes in Fig. 4(b) was calculated, as shown in Fig. 4(c). The profile includes two absorption peaks that correspond to the two blood-filled tubes. The FWHMs of the two tubes were estimated to be 0.44 mm and 0.68 mm, respectively, which are close to the inner tube sizes. Several sources may contribute to the slightly larger sizes measured from the images. The tube wall may generate photoacoustic signals and positions of tubes may be slightly tilted from the perpendicular direction of the light beam and ultrasound receiving axis. To further access the feasibility of the system for biomedical applications, a mouse ear was imaged ex vivo. Figure 5 shows the PAM MAP image of the mouse ear. The major blood vessels and network pattern of the mouse ear were clearly resolved.
Fig. 4.
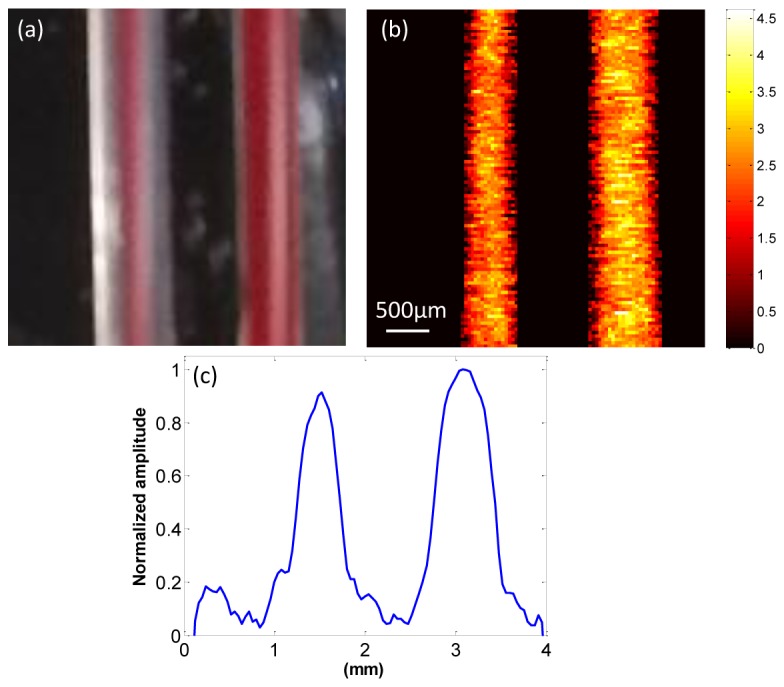
(a) Photograph of two polyethylene tubes filled with rat blood; (b) corresponding PAM images of blood tubing; (c) averaged normalized cross-sectional profile of the blood tubes in (b).
Fig. 5.
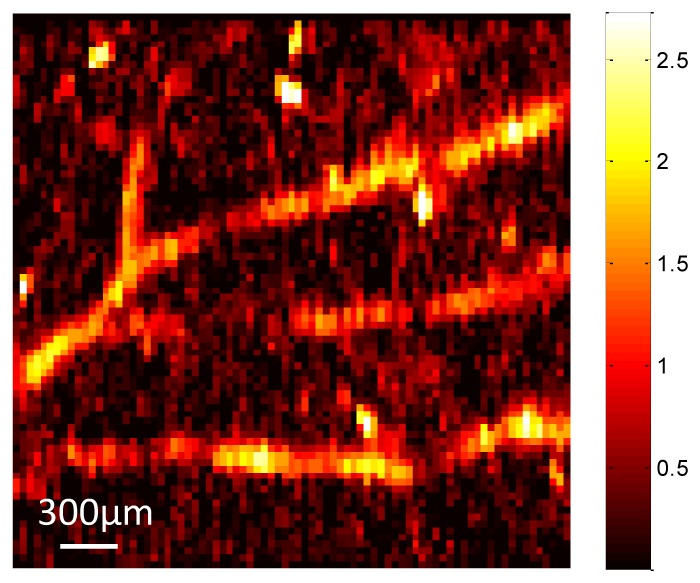
PAM image of a mouse ear.
The feasibility of laser-diode OR-PAM system for biomedical applications has been demonstrated by imaging the mouse ear. Additionally, two different sizes of polyethylene tubing filled with rat blood were imbedded in ultrasound gel and imaged to demonstrate the capability of the low-cost PAM system of imaging blood vessels. The pulsed laser diode combined with the driver board cost only $600 dollars. The cost and the size of the PAM system has therefore been significantly reduced. In addition, the 1 KHz pulse repetition rate increased the data acquisition speed, and also allowed more averages to increase the SNR. In this study, 128 pulses were averaged to compromise the image quality and the data acquisition speed. More averages can be performed to further improve the SNR. The PAM image quality of the mouse ear shown in Fig. 5 was limited by several factors. The pulse width of the laser diode was 124 ns, which was limited by the driver board. Assuming the speed of sound is 1540 m/s in biological tissue, the stress confinement is not stringently satisfied when the blood vessels are smaller than 191 µm. This will affect the efficiency of acoustic wave generation. As a result, the initial pressure rise from blood vessels that are smaller than 191 µm could be affected. Besides, since the mouse ear was imaged ex vivo, some blood was lost during the incision of the mouse ear, and the optical absorption of the blood vessels was also degraded compared with in vivo mouse ear imaging. The pulse energy on the imaged sample was 3 µJ. Assuming that the laser beam focuses at 200 µm below the sample surface, the estimated energy density on the surface is 2.5 mJ/cm2, which is far below the maximum permissible exposure (MPE) according to American National Standards Institute (ANSI) safety standard. A pulsed laser diode that can achieve a shorter pulse width of 30 ns and higher peak power of 650 W has recently been made commercially available. In future studies, smaller size blood tubes immersed in intralipid solution or embedded in tissue phantoms as well as ex vivo and in vivo tissue samples will be imaged with the upgraded pulsed laser diode to improve the image quality and resolve more detailed microvasculature distribution. Future efforts will also be devoted to developing the portable hand-held PAM probe for in vivo clinical applications.
4. Summary
In this paper, a high resolution OR-PAM system using a pulsed laser diode as an excitation source has been developed. The lateral resolution of the PAM was estimated to be 7 µm by imaging the carbon fiber. Blood tubing and mouse ear were imaged to demonstrate the feasibility of the laser-diode-based PAM to image biological tissue. This compact and low-cost PAM with laser diode excitation has demonstrated great potential for clinical applications.
Acknowledgments
This research was supported by NIH R01CA151570. The authors thank Feifei Zhou and Mohsen Erfanzadeh from Optical and Ultrasound Imaging Lab at the University of Connecticut for helping with the biological tissue preparation and experiment. The authors thank Prof. Lvming Zeng, Key Lab of Optic-Electronic and Communication, Jiangxi Science and Technology Normal University, China, for his valuable suggestions on how to handle this laser diode.
References and links
- 1.Xu M., Wang L. V., “Photoacoustic imaging in biomedicine,” Rev. Sci. Instrum. 77(4), 041101 (2006). 10.1063/1.2195024 [DOI] [Google Scholar]
- 2.Wang L. V., Hu S., “Photoacoustic tomography: in vivo imaging from organelles to organs,” Science 335(6075), 1458–1462 (2012). 10.1126/science.1216210 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Beard P., “Biomedical photoacoustic imaging,” Interface Focus 1(4), 602–631 (2011). 10.1098/rsfs.2011.0028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Karabutov A., Savateeva E. V., Podymova N. B., Oraevsky A. A., “Backward mode detection of laser-induced wide-band ultrasonic transients with optoacoustic transducer,” J. Appl. Phys. 87(4), 2003–2014 (2000). 10.1063/1.372127 [DOI] [Google Scholar]
- 5.Wang X., Pang Y., Ku G., Xie X., Stoica G., Wang L. V., “Noninvasive laser-induced photoacoustic tomography for structural and functional in vivo imaging of the brain,” Nat. Biotechnol. 21(7), 803–806 (2003). 10.1038/nbt839 [DOI] [PubMed] [Google Scholar]
- 6.Wang T., Kumavor P. D., Zhu Q., “Application of laser pulse stretching scheme for efficiently delivering laser energy in photoacoustic imaging,” J. Biomed. Opt. 17(6), 061218 (2012). 10.1117/1.JBO.17.6.061218 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Yao J., Wang L. V., “Photoacoustic microscopy,” Laser Photon. Rev. 7(5), 758–778 (2013). 10.1002/lpor.201200060 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hu S., Wang L. V., “Optical-resolution photoacoustic microscopy: auscultation of biological systems at the cellular level,” Biophys. J. 105(4), 841–847 (2013). 10.1016/j.bpj.2013.07.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zhang H. F., Maslov K., Stoica G., Wang L. V., “Functional photoacoustic microscopy for high-resolution and noninvasive in vivo imaging,” Nat. Biotechnol. 24(7), 848–851 (2006). 10.1038/nbt1220 [DOI] [PubMed] [Google Scholar]
- 10.Maslov K., Zhang H. F., Hu S., Wang L. V., “Optical-resolution photoacoustic microscopy for in vivo imaging of single capillaries,” Opt. Lett. 33(9), 929–931 (2008). 10.1364/OL.33.000929 [DOI] [PubMed] [Google Scholar]
- 11.Zemp R. J., Song L., Bitton R., Shung K. K., Wang L. V., “Realtime photoacoustic microscopy in vivo with a 30-MHz ultrasound array transducer,” Opt. Express 16(11), 7915–7928 (2008). 10.1364/OE.16.007915 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wang L. V., “Multiscale photoacoustic microscopy and computed tomography,” Nat. Photonics 3(9), 503–509 (2009). 10.1038/nphoton.2009.157 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rao B., Li L., Maslov K., Wang L. V., “Hybrid-scanning optical-resolution photoacoustic microscopy for in vivo vasculature imaging,” Opt. Lett. 35(10), 1521–1523 (2010). 10.1364/OL.35.001521 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hu S., Maslov K., Wang L. V., “Second-generation optical-resolution photoacoustic microscopy with improved sensitivity and speed,” Opt. Lett. 36(7), 1134–1136 (2011). 10.1364/OL.36.001134 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wang L., Maslov K., Yao J., Rao B., Wang L. V., “Fast voice-coil scanning optical-resolution photoacoustic microscopy,” Opt. Lett. 36(2), 139–141 (2011). 10.1364/OL.36.000139 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hajireza P., Shi W., Zemp R. J., “Real-time handheld optical-resolution photoacoustic microscopy,” Opt. Express 19(21), 20097–20102 (2011). 10.1364/OE.19.020097 [DOI] [PubMed] [Google Scholar]
- 17.Zhang C., Maslov K., Hu S., Chen R., Zhou Q., Shung K. K., Wang L. V., “Reflection-mode submicron-resolution in vivo photoacoustic microscopy,” J. Biomed. Opt. 17(2), 020501 (2012). 10.1117/1.JBO.17.2.020501 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Guo Z., Favazza C., Garcia-Uribe A., Wang L. V., “Quantitative photoacoustic microscopy of optical absorption coefficients from acoustic spectra in the optical diffusive regime,” J. Biomed. Opt. 17(6), 066011 (2012). 10.1117/1.JBO.17.6.066011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chen S. L., Xie Z., Ling T., Guo L. J., Wei X., Wang X., “Miniaturized all-optical photoacoustic microscopy based on microelectromechanical systems mirror scanning,” Opt. Lett. 37(20), 4263–4265 (2012). 10.1364/OL.37.004263 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zhang X., Zhang H. F., Jiao S., “Optical coherence photoacoustic microscopy: accomplishing optical coherence tomography and photoacoustic microscopy with a single light source,” J. Biomed. Opt. 17(3), 030502 (2012). 10.1117/1.JBO.17.3.030502 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Yao J., Wang L., Li C., Zhang C., Wang L. V., “Photoimprint photoacoustic microscopy for three-dimensional label-free subdiffraction imaging,” Phys. Rev. Lett. 112(1), 014302 (2014). 10.1103/PhysRevLett.112.014302 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Xie Z., Roberts W., Carson P., Liu X., Tao C., Wang X., “Evaluation of bladder microvasculature with high-resolution photoacoustic imaging,” Opt. Lett. 36(24), 4815–4817 (2011). 10.1364/OL.36.004815 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wang T., Yang Y., Alqasemi U., Kumavor P. D., Wang X., Sanders M., Brewer M., Zhu Q., “Characterization of ovarian tissue based on quantitative analysis of photoacoustic microscopy images,” Biomed. Opt. Express 4(12), 2763–2768 (2013). 10.1364/BOE.4.002763 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Allen T. J., Beard P. C., “Pulsed near-infrared laser diode excitation system for biomedical photoacoustic imaging,” Opt. Lett. 31(23), 3462–3464 (2006). 10.1364/OL.31.003462 [DOI] [PubMed] [Google Scholar]
- 25.Kolkman R. G. M., Steenbergen W., van Leeuwen T. G., “In vivo photoacoustic imaging of blood vessels with a pulsed laser diode,” Lasers Med. Sci. 21(3), 134–139 (2006). 10.1007/s10103-006-0384-z [DOI] [PubMed] [Google Scholar]
- 26.Maslov K., Wang L. V., “Photoacoustic imaging of biological tissue with intensity-modulated continuous-wave laser,” J. Biomed. Opt. 13(2), 024006 (2008). 10.1117/1.2904965 [DOI] [PubMed] [Google Scholar]
- 27.Zeng L., Liu G., Yang D., Ji X., “3D-visual laser-diode-based photoacoustic imaging,” Opt. Express 20(2), 1237–1246 (2012). 10.1364/OE.20.001237 [DOI] [PubMed] [Google Scholar]
- 28.LeBoulluec P., Liu H., Yuan B., “A cost-efficient frequency-domain photoacoustic imaging system,” Am. J. Phys. 81(9), 712–717 (2013). 10.1119/1.4816242 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zeng L., Liu G., Yang D., Ji X., “Portable optical-resolution photoacoustic microscopy with a pulsed laser diode excitation,” Appl. Phys. Lett. 102(5), 053704 (2013). 10.1063/1.4791566 [DOI] [Google Scholar]
- 30.Aguirre A., Guo P., Gamelin J., Yan S., Sanders M., Brewer M., Zhu Q., “Co-registered 3-D ultrasound and photoacoustic imaging system for ovarian tissue characterization,” J. Biomed. Opt. 14(5), 054014 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]


