Abstract
Infectious diseases such as norovirus can induce emesis (vomiting), which can be of a projectile nature. Although studies have been carried out on transmission, prevalence and decontamination of such micro-organisms within various environments, little is known about the extent to which the surrounding environment is contaminated when an individual vomits. This is an important consideration for infection control purposes. The aim of this study was to develop a simulated vomiting system (Vomiting Larry) to be used for assessing the extent to which projected fluid can contaminate the environment. Vomiting Larry was set up within a Controlled Atmosphere Chamber (CAC) facility at the Health and Safety Laboratory (HSL). Simulated vomiting was undertaken using water as a vomitus substitute containing a fluorescent marker enabling small splashes, ordinarily missed, to be visualised using UV lighting. Experiments revealed that splashes and droplets produced during an episode of projectile vomiting can travel great distances (>3 m forward spread and 2.6 m lateral spread). The research highlighted that small droplets can be hard to see and therefore cleaning all contaminated surfaces is difficult to achieve. Evidence from this study suggests that areas of at least 7.8 m2 should be decontaminated following an episode of projectile vomiting.
Keywords: Emesis, environmental contamination, infection control, norovirus, vomiting, Vomiting Larry
Introduction
Emesis is important in terms of ridding the upper gastrointestinal tract of toxins and pathogens. The act of vomiting by an individual infected with an infectious disease such as norovirus disseminates the agent into the local environment. Electron microscopic studies have suggested that as many as 3×107 norovirus particles could be distributed into the immediate surroundings following an episode of vomiting, based on a bolus of 30 ml i.e. 1×109 virus particles/L (Caul, 1995). The robust nature of many infectious agents allows them to survive in the environment for protracted periods of time, particularly when associated with organic matter such as skin squames, faeces and vomit. This increases the likelihood of infection transmission if they are not removed from the environment. Cleaning and decontamination of surfaces contaminated with an infectious agent post-emesis is therefore an important aspect of infection control.
Research relating to the physiology of vomiting has been carried out, although most published studies relating to the mechanics of vomiting date back to the early 1900s. Such studies have demonstrated that the mechanism of emesis in humans and other mammals is complex (Lumsden and Holden, 1969) and is still controversial (Pickering and Jones, 2002). It is accepted that the process comprises three key phases, which occur in the following order: nausea, retching and vomiting. Projectile vomiting (sometimes referred to as forced vomiting) often occurs suddenly and without warning, i.e. without nausea and retching. Consequently an individual may have an episode of projectile vomiting at any time, e.g. while standing in a queue, on public transport, or in a restaurant or hospital bed.
Based on the widely accepted text by Guyton and Hall (2011), vomiting is initiated via a deep inspiratory breath, which allows the upper oesophageal sphincter to open, closing of the glottis and posterior nares, as well as a strong downward contraction of the diaphragm. Contraction of all abdominal muscles occurs simultaneously, consequently squeezing the stomach between the two sets of muscles, i.e. the diaphragm and the abdominals, creating a high level of intragastric pressure. The lower oesophageal sphincter at the top of the stomach then relaxes, as does the perioesophageal (crural) portion of the diaphragm required to release the fluid into the oesophagus and out of the mouth (Guyton and Hall, 2011). Many early studies have shown reasonably conclusively that the role of the stomach wall during emesis is minimal (Haskell, 1924).
Projectile vomiting is likely to have the biggest impact in terms of splash distribution and provides a worst-case scenario for cleaning and disinfection following vomiting. This study describes the development of a novel simulated vomiting system (Vomiting Larry) to simulate projectile vomiting. This system would then enable research into environmental contamination and aid development and implementation of infection control practices.
Methods
In order to develop a simulated vomiting system, key pieces of information regarding vomiting were required. These were typical distances travelled by ejected vomitus fluid, the flow rate or intragastric pressure exerted during an episode of projectile vomiting, and the typical volumes of fluid produced.
Information regarding the distance fluid travelled during an episode of vomiting is sparse. One study by Brimacombe and Keller (2006) established that a patient who had an episode of projectile vomiting during the testing of a laryngeal mask airway achieved a distance of 1.2 metres.
Evidence relating to the velocity or flow rate of emitted fluid during vomiting was not found. Research regarding intragastric pressure during the involuntary process of projectile vomiting is sparse. One study by Iqbal et al (2008) showed that intragastric pressure changes during a range of activities including vomiting. This study used a transnasally placed manometry catheter to measure intragastric pressure and found that the maximum intragastric pressure exerted during vomiting was 38.66 kPa, with a mean of 10.93 kPa after 40 episodes of vomiting from 10 volunteers. This research was used as a starting point in the development of the model with regards to pressure for the system, in an attempt to expel the main bulk of the fluid a distance of 1.2 metres.
The volume of fluid produced through projectile vomiting is likely to vary from person to person and the volume of digester, and there are limited data on volumes of vomit emitted; none have been identified for symptomatic norovirus patients. A study by Saetta and Quinton (1991) on the residual gastric content after gastric lavage and ipecacuanha-induced emesis in self-poisoned patients showed that the volumes of vomitus produced from 13 ipecacuanha-treated patients varied between 0.4 and 1.35 L. Based on this information, it was decided that for this study, a volume of 1 L would be used.
The simulated vomiting system was therefore required to hold 1 L of fluid, which was to be ejected in a similar fashion to that of a human during an episode of projectile vomiting. The bulk of the expelled fluid was to land 1.2 m from the outlet source possibly using a pressure of 10.93 kPa.
Development of Vomiting Larry
Projectile vomiting can occur without warning, and thus the simulated vomiting system was designed to represent a person projectile vomiting whilst in a standing position. The stomach of the system encompassed a plastic cylinder 3 mm thick, 95 mm in diameter to the outer edge and 300 mm in length to comfortably hold 1 L of fluid. The base of the cylinder was attached to a double acting actuator (pneumatic ram, PRA/182063/M/250, Norgren Ltd.), which would operate in a piston pump fashion to force the fluid out of the cylinder by forcing compressed air in at the base of the actuator to push the piston up the shaft of the cylinder to force the fluid out. A stainless steel disc (91.5 mm diameter × 15 mm thick) was attached to the top of the piston head, i.e. at the top of the actuator. A rubber seal (6 mm thick) was placed around the edge of the disc to ensure a snug fit into the cylinder and prevent fluid (i.e. simulated vomit) leaking from the cylinder into the actuator. Two stainless steel cuffs (10 mm thick) were located 100 mm from the top and bottom of the cylinder for support during simulated vomiting. The base and top of the cylinder were fitted with stainless steel plates (120 mm × 120 mm × 20 mm). The base plate comprised a centrally located hole (65 mm diameter) to allow the piston of the actuator to move freely allowing the piston head to move up and down the cylinder. The top plate contained a 120 mm long stainless steel outlet tube with an aperture (20 mm outer diameter) that would be connected to a representative oesophagus. The steel cap also had a tubeless 20 mm aperture to allow the cylinder to be filled with fluid. Once the cylinder was filled with fluid the aperture would be closed by means of a screw fixture. The cylinder and actuator were fixed to a stainless steel plate (102 mm × 935 mm × 25 mm) to support the system during the simulated vomiting process.
An authentic manikin head (Airway Larry, Simulaids, Inc.) was used for the head of the simulated vomiting system. Airway Larry is an adult airways management trainer, which simulates non-anaesthetised patients. This trainer is used for practising intubation, ventilation, suction and CPR techniques. The manikin has realistic anatomy and key structures including teeth, tongue, oral and nasal pharynx, larynx, epiglottis, arytenoids, false cords, true vocal cords, trachea, lungs, oesophagus and stomach.
The lungs and stomach in Airway Larry were plastic attachments and not necessary for this research. The ends of the tubes representing the primary bronchi were blocked with 16 mm diameter stainless steel blanks fixed in place by hose clips. The tube representing the oesophagus from the manikin head was attached to the outlet tube on top of the cylinder and fixed by means of a hose clip. The piston pump and manikin head were then mounted to a wooden frame made from 18 mm thick plywood, which allowed the whole unit to be freestanding. On completion, the simulated vomiting system (now termed Vomiting Larry) stood 1.6 m from the floor to the top of the manikin head (Figure 1).
Figure 1.
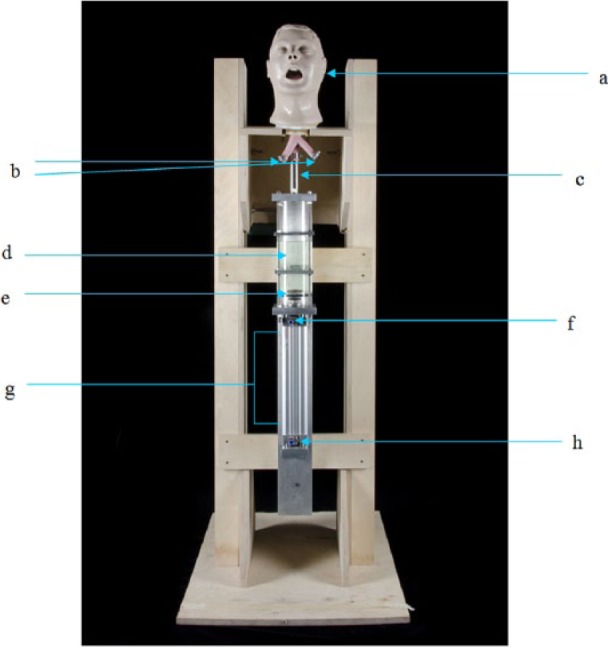
The simulated vomiting system (Vomiting Larry)
a: Airway Larry
b: Blocked off bronchioles of Airway Larry
c: Outlet pipe connected to Airway Larry
d: Cylinder containing 1 L of fluid
e: Piston
f: Air inlet to push piston down
g: Pneumatic ram
h: Air inlet to push piston up
Simulated projectile vomiting
The simulated vomiting system was set up in a Controlled Atmosphere Chamber (CAC). This chamber is a sealed 35 m3 unit with air inlet ducts in the ceiling and HEPA-filtered air outlet air ducts in the floor below walking level. The walking floor level is a raised grid bed several inches above the air outlet ducts. The CAC’s air handling is controlled remotely and incorporates a temperature and relative humidity regulation system, which can be altered as required. In this instance the air handling was set to standard room temperature (25oC) and relative humidity (40%). The air handling system was then switched off and the CAC isolated via damper closure prior to vomiting simulation so as to prevent any air movement from altering the distribution of the spray of droplets and aerosols produced from the system.
In order to assess the distance travelled by the expelled fluid the CAC floor was covered with safety vinyl followed by two layers of plastic sheeting, which were taped to the base of the walls of the CAC so as to contain the fluid. A grid system was then prepared using masking tape to mark out 20 cm × 20 cm squares. The simulated vomiting system was positioned mid-way along one wall of the chamber, facing into the chamber, which allowed an area of 3.1 m × 2.6 m into which the fluid would be expelled. Plastic tubing (5 mm inner diameter, 1.5 mm thick) was connected to the laboratory plant air supply through a pressure regulator (P3HEA12ESMBNGB standard filter/regulator, Parker Hannifin Corp) and solenoid valve (5/2 mechanical valve ¼ port series VFM 350, SMC) and finally attached to the actuator air inlet ports on the simulated vomiting system.
To enable visibility of small splashes of projected fluid, 1 g of fluorescent powder (Tinopal®, Ciba Inc.) was dissolved in 1 L of water. The fluid was added to the cylinder of the system. Three tripods each mounting four black light UV bulbs (T8 18W 600 mm, Philips) housed within UV filter cases (custom made MUG-2 UV filters, Schott AG Manufacturers) were set up in three corners of the CAC. Once set up, the actuator was triggered via the solenoid valve switch, which forced air under the base of the piston pushing it up the length of the cylinder forcing fluid into the oesophageal tube of Airway Larry and out of the mouth.
As the only published information on vomiting was possible volume (1 L, Saetta and Quinton, 1991), potential distance covered (1.2 m, Brimacombe and Keller, 2006) and intragastric pressure required (10.93 kPa, Iqbal et al, 2008), several simulated vomiting trials were undertaken in order to ascertain the actual amount of pressure required for the bulk of the fluid to travel a distance of 1.2 m. Post-simulated vomiting, the CAC was left for 15 minutes to allow any aerosols produced to settle out before entering the chamber to photograph the spread of fluid across the grid floor. Once this distance had been achieved, further experiments were undertaken to visually assess the extent of the spread of splash and droplets. The visual experiments were repeated in triplicate and photographs taken to identify distance of spread.
Results
A simulated vomiting system (Vomiting Larry) was developed successfully (Figure 1) and used to assess the amount to which the local environment is contaminated after an episode of projectile vomiting. The initial 10.33 kPa intragastric pressure described by Iqbal et al, (2008) was insufficient to move the piston of the actuator. Considerable air pressure (800 kPa) was required for this simulated projectile vomiting system to expel the main bulk of the fluid (1 L) a distance of 1.2 m from the source, i.e. mouth of the system.
Film footage and photographs taken from three simulated projectile vomiting experiments carried out using 1 L of water (plus fluorescent marker) and 800 kPa of pressure to eject the bulk fluid 1.2 m highlighted fluid dynamics during vomiting, splash upon impact with the floor and total distance travelled by droplets.
Sequential images of simulated projectile vomiting are shown in Figure 2. Many droplets were released from Vomiting Larry prior to the appearance of the main bulk of the fluid (Figure 2a), which were projected forwards and outwards from the orifice. As the main bulk of the fluid exited the mouth, fluid collided creating ‘strings’ of fluid and droplets, some of which were directed back towards the system (Figure 2b). Droplets tended to fall out as they lost momentum, which created a cloud of droplets beneath and around the main flow of the fluid (Figure 2b). During mid-flow, the bulk fluid appeared more directional, with less droplet formation (Figure 2c and d). Towards the end of the simulated vomiting episode, the fluid travelled less far before fallout occurred, as the pressure decreased (Figure 2e).
Figure 2.
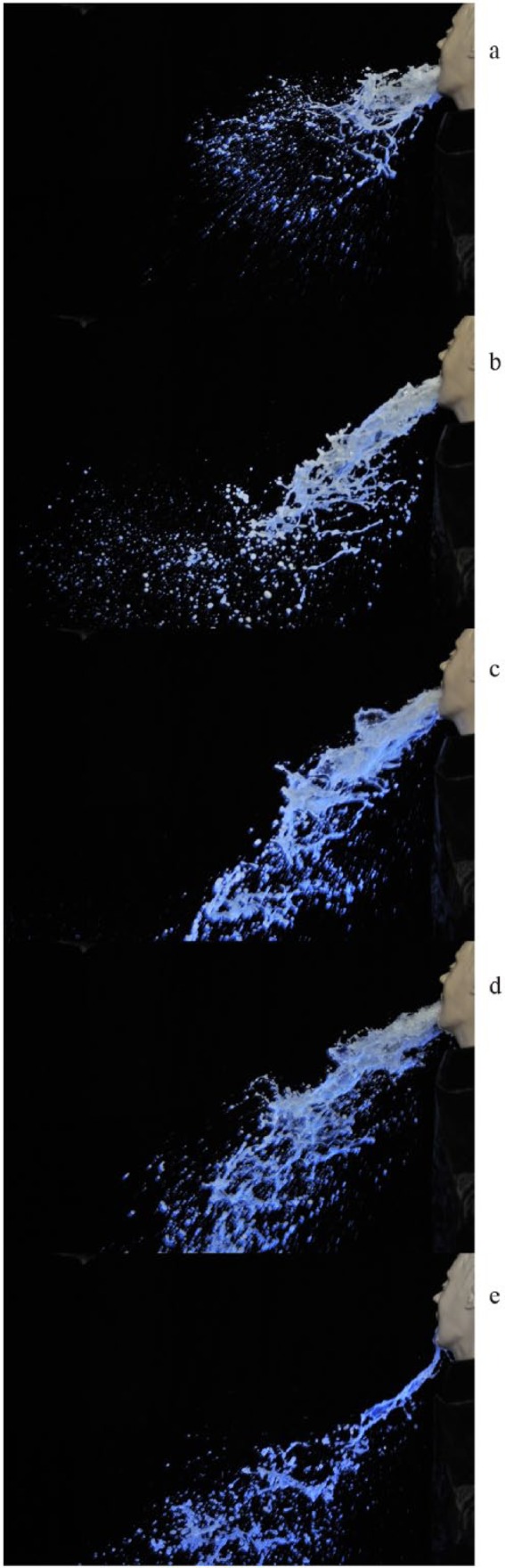
Images (a–-e) are sequential photographs of simulated vomiting taken 10 ms apart under UV light.
a: Release of droplets prior to main bulk fluid
b: ‘Strings’ of fluid and droplet fallout
c: Mid flow of fluid
d: Less droplet formation
e: Fluid travelling less far towards end of simulation
Figure 3 highlights fluid rebound from the floor after the initial impact. Splash and droplets were created by the rebounding fluid, which spread further still from the main impact site and/or collided with the contra flow of fluid still being projected from the mouth of the system to the floor. Impacted fluid continued to move during and after simulated vomiting (Figure 3a). Once settled, the main bulk of the fluid filled an area 1.2 m × 1.6 m. For each experiment splash was identified on the wall directly opposite the front of the system and on the two adjacent walls covering a total distance >3 m longitudinal spread and >2.2 m lateral spread. The full extent to which the fluid was distributed is illustrated in Figure 4. Examination of the expelled fluid on the floor of the CAC revealed that only the main bulk of the liquid and major splashes were visible under standard white lighting. Smaller splashes and droplets were difficult to identify without the aid of UV lighting.
Figure 3.

Rebounding fluid (highlighted at A) from the initial impact during simulated vomiting (scale: grid sections = 20 cm x 20 cm) creates widespread splash.
a: Rebounding splash upon fluid impact with floor surface
Figure 4.
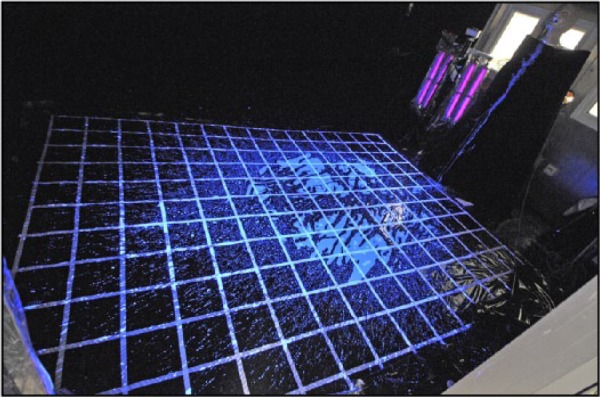
Spread of splash post-simulated vomiting (scale: grid sections = 20 cm x 20 cm)
Discussion
This study describes the development of a novel simulated vomiting system (Vomiting Larry), which will be used to assess further the contamination of the local environment after an episode of vomiting. This system can be used as a training aid for cleaning and disinfection practices as part of an infection prevention and control strategy. Modifications to Vomiting Larry would also allow this system to simulate coughing and sneezing that could be used to investigate routes of transmission from individuals infected with a variety of pathogens ultimately increasing our knowledge of infection and its control.
The development of Vomiting Larry has shown that splashes and droplets produced as a result of simulated projectile vomiting can travel a considerable distance. Given that much of the smaller splashes and droplets were not visible under standard white lighting, it would be difficult to clean up all potentially contaminated splashes from surfaces that have become contaminated after an episode of vomiting from a symptomatic patient infected with an infectious agent such as norovirus solely based on visual observation of area affected. In confined spaces contamination will spread to vertical surfaces away from the obvious source of contamination. Therefore, members of staff involved in cleaning surfaces after vomiting has occurred need to be aware of the likely surface area of contamination.
It has been shown that many infectious particles can be contained within vomit (Caul, 1995). Vomiting Larry could be employed to assess the true extent of dissemination of pathogens into the local environment through vomiting, by means of adding model surrogates of infectious agent such as norovirus to the system. This would demonstrate how well the agent survives the vomiting process, e.g. shearing forces and drying out, as well as identifying how far the viable agent could spread and whether it is in sufficient concentrations to be infectious upon contact with a susceptible host. Information gathered from future research could then be built into a fluid model to highlight the dynamics of vomitus spread and associated pathogens as well as its impact in a range of settings.
This study used 1 L of water as a vomitus substitute based on the research by Saetta and Quinton (1991) to allow for a worst-case scenario in terms of fluid spread due to being a lower density than vomit. It also utilised safety vinyl floor material covered with two layers of plastic sheeting, which would likely create a larger amount of splash and possibly achieve greater distances of travel compared with softer surfaces such as carpet. Vomiting Larry could be employed in further research to compare the spread of expelled fluid of differing volumes and viscosities as well as to assess differences in the amount of spread from rebounding droplets and splash when landing on alternative surfaces such as carpet. The physiochemical properties of stomach digestion also need to be considered.
Another key consideration in terms of environmental contamination and subsequent infection transmission is body position of the emetic individual, who may be lying down, bent over, kneeling, sitting, or standing. This could be investigated using the simulated vomiting system developed here.
In addition, simulated vomiting was conducted within an empty 35 m3 chamber. Studies have described cases of infectious diseases being spread through vomiting in various settings such as restaurants and aeroplanes for instance where close proximity obstacles such as tables, seats and even fellow human beings would alter the trajectory of the vomitus flow (Widdowson et al, 2005; Baker et al, 2010). Furniture and manikins could also be added into the CAC to investigate how such obstacles affect the spread of projected fluid and therefore how this might affect clean up procedures and disease transmission.
From the images and film footage gathered in this current study, simulated vomiting produced droplets and potentially aerosols. Further tests are required in order to assess whether simulated projectile vomiting generates aerosols. Mathematical models based on epidemiological research have shown that norovirus transmission is possible via inhalation of infectious aerosols produced during an episode of vomiting from an infected individual (Leung et al, 2006). The inhaled aerosols are then ingested by the susceptible host so as to elicit infection. The size and numbers of droplets and aerosols produced from vomiting, including projectile vomiting, remains unknown. A particle counter could be used to measure the numbers and size range of particles generated during simulated projectile vomiting. This would achieve a better understanding of the potential for infection transmission from infectious aerosols.
The generally accepted process by which the body releases vomitus fluid from the stomach is by increasing the intragastric pressure through simultaneous contraction of the thoracic and abdominal muscles (Guyton and Hall, 2011). This essentially creates a pressure vessel of the stomach contents before the oesophageal sphincter opens to allow the release of stomach contents into the oesophagus and out of the mouth. This differs slightly from the piston pump arrangement of the simulator, which pushed the fluid out of the stomach into the oesophagus and out of the mouth. This mechanistic difference may be the reason for the considerable 800 kPa pressure requirement of Vomiting Larry to project fluid a distance of 1.2 m compared with that noted by Iqbal et al (2008). Resources at the time of research demanded a simpler mechanism for this prototype. This system could be altered to incorporate a specialised and certified pressure vessel that specifically represented intragastric pressure of the stomach and contents as created by the thoracic and abdominal muscles, therefore more closely representing human physiology.
In the system developed here, it is likely that increased pressure was required to overcome the mechanical weight and friction within the system, i.e. the weight of the piston, and friction created by the stainless steel piston rubbing against the inner walls of the plastic cylinder (representative stomach). Stiction, that is, the force required to move the fluid through the system (comprised of a plastic cylinder stomach, and stainless steel and rubber oesophagus) is also likely to be greater than that of the natural human system where the stomach, oesophageal and oral epidermal surfaces are flexible and well lubricated. It is also worth noting that although 800 kPa of pressure was measured at the supply source, pressure losses would occur through the system before reaching the piston. Nonetheless, the simulated vomiting system (Vomiting Larry) described here successfully expelled 1 L of fluid 1.2 m as reported in the literature (Saetta and Quinton, 1991; Brimacombe and Keller, 2006) and enabled the examination of the extent to which the local environment becomes contaminated after an episode of projectile vomiting.
Acknowledgments
The author would like to thank the Department of Health and the Investment Research Programme of the Health & Safety Laboratory (HSL) for funding this work and publication as well as the Centre for Workplace Health, Visual Presentation Service, the Occupational Hygiene Unit, Workshops and Karen Wilkinson at HSL for their contributions to the production of Vomiting Larry and film footage. The author is also grateful to Drs Mary Trainor, Andrew Curran, Alan Beswick and Jayne Farrant at HSL for their support of the work. The author also wishes to thank Drs Gareth Evans, Jayne Farrant of HSL, and Karen Stanley and Professor Nicola Woodroofe of Sheffield Hallam University for proofreading the manuscript.
Footnotes
© Crown Copyright (2013): This is independent research commissioned and co-funded by the Policy Research Programme in the Department of Health and the Health & Safety Laboratory. The views expressed are not necessarily those of the Department of Health.
Declaration of conflicting interest: The author declares that there is no conflict of interest.
Funding: This work was supported by the Department of Health and the Investment Research Programme of the Health & Safety Laboratory.
References
- Baker K, Morris J, McCarthy N, Saldana L, Lowther J, Collinson A, Young M. (2010) An outbreak of norovirus infection linked to oyster consumption at a UK restaurant, February 2010. Journal of Public Health 33(2): 205–11. [DOI] [PubMed] [Google Scholar]
- Brimacombe J, Keller C. (2006) Hypopharyngeal seal pressure during projectile vomiting with the ProSeal laryngeal mask airway: a case report and laboratory study. Canadian Journal of Anaesthesia 53(3): 328. [DOI] [PubMed] [Google Scholar]
- Caul EO. (1995) Hyperemesis hiemis – a sick hazard. Journal of Hospital Infection 30(Supplement): 498–502. [DOI] [PubMed] [Google Scholar]
- Guyton AC, Hall JE. (2011) Textbook of medical physiology, 12th edn Saunders Elsevier Inc: Amsterdam: 803–5. [Google Scholar]
- Haskell RC. (1924) The physiology of vomiting. Journal of the National Medical Association 16(1): 8–10. [PMC free article] [PubMed] [Google Scholar]
- Iqbal A, Haider M, Stadlhuber RJ, Karu A, Corkill S, Filipi CJ. (2008) A study of intragastric and intravesicular pressure changes during rest, coughing, weight lifting, retching, and vomiting. Surgical Endoscopy 22(12): 2571–5. [DOI] [PubMed] [Google Scholar]
- Leung TF, Cheng FWT, Lai RWM, Chan PKS, Chan RFY, Li CK, Ng PC. (2006) Infection control for norovirus gastroenteritis outbreak in acute open-designed paediatric ward. 6th International Conference of Hospital Infection Society, Amsterdam, The Netherlands, 15-18 October 2006 S76. [Google Scholar]
- Lumsden K, Holden WS. (1969) The act of vomiting in man. Gut 10(3): 173–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pickering M, Jones JF. (2002) The diaphragm: two physiological muscles in one. Journal of Anatomy 201(4): 305–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saetta JP, Quinton DN. (1991) Residual gastric content after gastric lavage and ipecacuanha-induced emesis in self-poisoned patients: an endoscopic study. Journal of the Royal Society of Medicine 84(1): 35–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Widdowson MA, Glass R, Monroe S, Beard RS, Bateman JW, Lurie P, Johnson C. (2005). Probable transmission of norovirus on an airplane. JAMA 293(15): 1859–60. [DOI] [PubMed] [Google Scholar]


