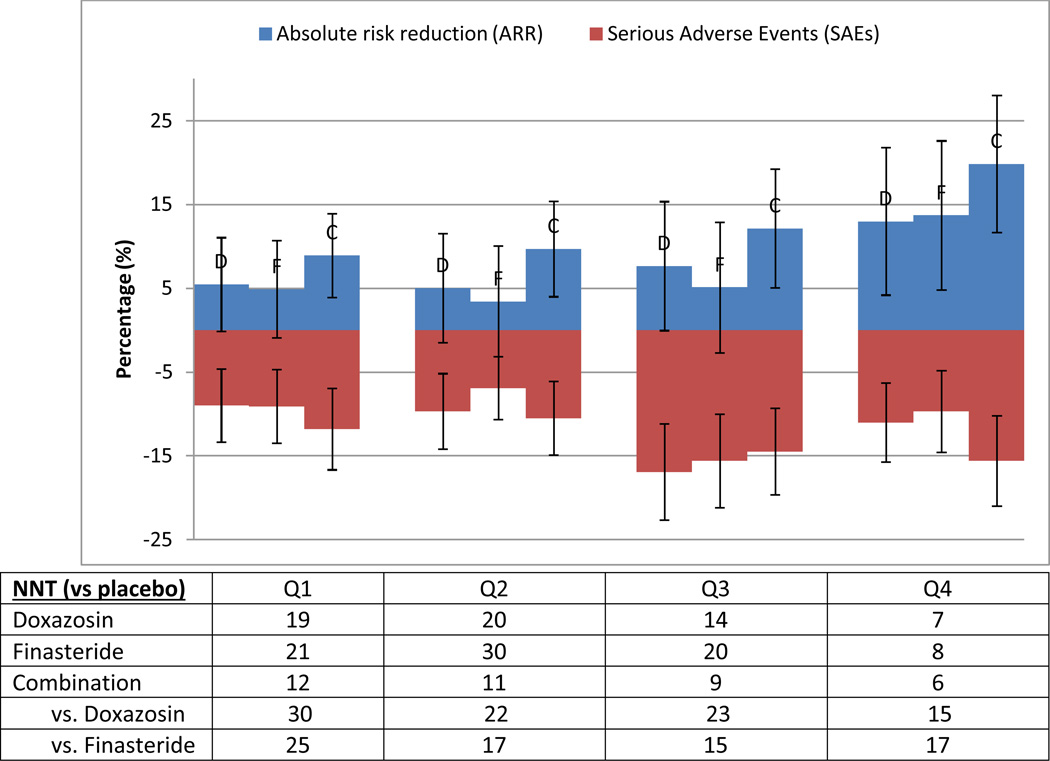
Figure 2. Medication treatment effects in terms of absolute risk compared to serious adverse events (SAEs) that resulted in therapy interruption/discontinuation.
Absolute risk reductions (with 95% CI) for the different treatment arms are calculated compared to the placebo arm and increase over quartiles of BPH progression, ordered from left to right (Q1->Q4), with each treatment arm reported (ordered as doxazosin (D), finasteride (F), combination (C) in each quartile). Serious adverse events (with 95% CI) are reported in raw percentages (listed as negative values for comparison). The number needed to treat (NNT) is derived from the absolute risk reductions in the respective groups, as listed in the table below.
ARR = absolute risk reduction compared to placebo; NNT = number needed to treat (1/ARR)