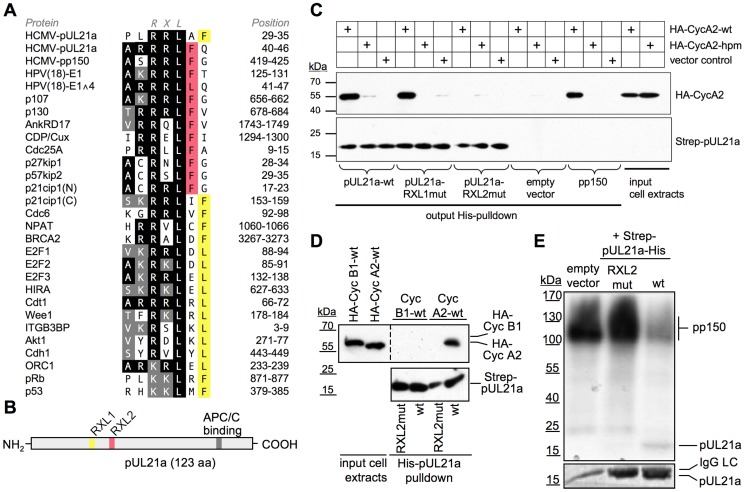
Figure 1. HCMV-pUL21a interacts with Cyclin A2.
(A) Alignment of two putative Cyclin A2-binding sites in pUL21a with validated RXL/Cy motifs of human Cyclin A2-CDK substrates and inhibitors. Identical residues are highlighted in black, conserved residues in grey, phenylalanine/leucin residues at positions +1 or +2 relative to the RXL motif in red or yellow. (B) Schematic of pUL21a showing the relative location of RXL sequence motifs, the APC/C-binding site and a minimal consensus CDK phosphorylation site (SP). (C, D) Recombinant Strep-His-tagged versions of pp150, pUL21a wild-type (wt), pUL21a-RRLAFARAAF (RXL1mut) and pUL21a-RRLFQARAFQ (RXL2mut) mutants were purified and immobilized to Ni-NTA agarose beads. The beads were incubated with HEK293 cell lysates containing ectopically expressed HA-Cyclin A2 wildtype (CycA2-wt), HA-Cyclin A2 hydrophobic patch mutant (CycA2-hpm) or HA-Cyclin B1 (CycB1). The input lysates and the pulled down material were analyzed by immunoblotting (IB) for the presence of pUL21a and HA-tagged cyclins. (E) Cyclin A2-associated kinase activity was immunoprecipitated from HEK293 lysates and incubated with the substrate protein pp150 and radioactively labeled γ-P32-ATP. Where indicated, equal amounts of pUL21a-wt or pUL21-RXL2mut were added to the reaction, as controlled by Coomassie staining (lower panel, IgG-LC: Immunoglobulin G light chain). The phosphorylation products were analyzed by autoradiography (upper panel).