Fig. 1.
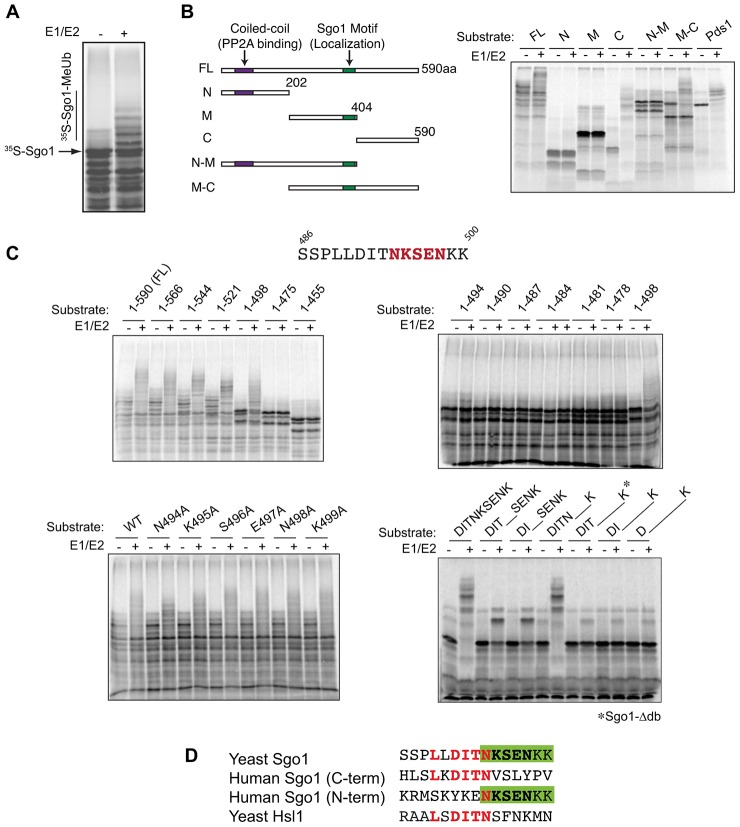
Budding yeast Sgo1 is an APC/C substrate, and its destruction depends on a non-canonical D-box. (A) Radiolabeled Sgo1 was translated in rabbit reticulocyte lysates and incubated with purified APC/C, ubiquitin and Cdh1, an APC/C activator. Purified E1 and E2 enzymes were added to promote ubiquitylation as indicated. Reaction products were analyzed by SDS-PAGE and autoradiography. Methylated ubiquitin was used to prevent polyubiquitin chain synthesis, which facilitates the clear detection of reaction products. The number of products generated in these reactions reflects the number of lysine residues that have been modified on the substrate, and modification of large numbers of lysine residues can lead to band heterogeneity and smearing. (B) Fragments of Sgo1 were used in APC/C ubiquitylation assays to identify the Sgo1 D-box. Radiolabeled fragments were translated in vitro and assayed as in A using a higher percentage polyacrylamide gel to resolve smaller fragments. Yeast securin (Pds1) was used as a positive control in the reactions at far right. FL, full-length Sgo1. (C) Various Sgo1 fragments and point mutants were used in APC/C assays, as in A, to further define the D-box. In the top two panels, fragments containing the indicated residues were translated from truncated PCR products. In the bottom two panels, reactions were carried out with translated full-length Sgo1 carrying the indicated point mutations or short deletions. An asterisk indicates the Sgo1-Δdb mutant. (D) Comparison of the budding yeast Sgo1 D-box to the Hsl1 D-box and the two APC/C degrons of human Sgo1 (Burton and Solomon, 2001; He et al., 2013; Karamysheva et al., 2009). Red text highlights similarities between all four sequences, and the green box highlights the similarities between C-terminal residues of the yeast Sgo1 D-box and the N-terminal degron of human Sgo1.