Fig. 4. Structure of the β sliding clamp.
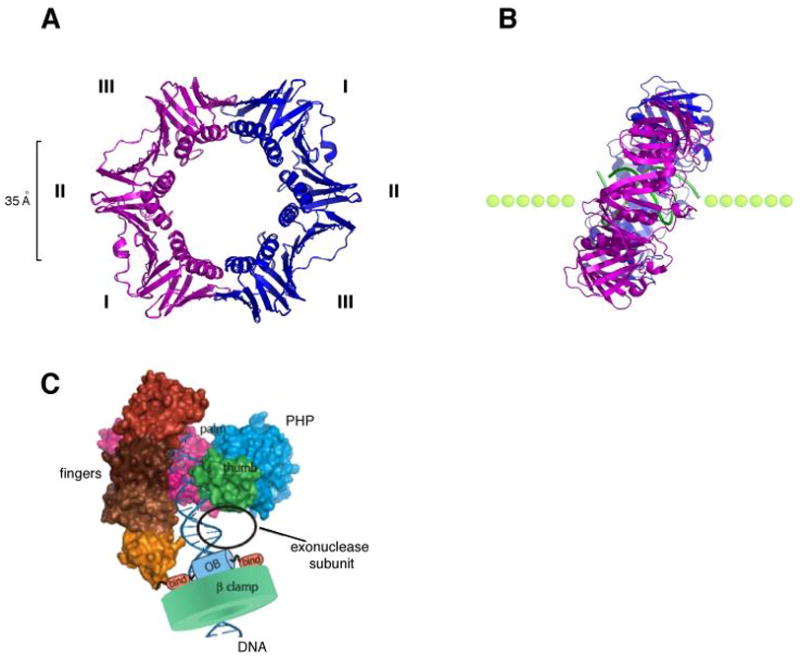
(A) Ribbon representation of the β homodimer (pdb code, 2pol). The two monomers (pink and blue) interact head-to-tail and form a highly symmetrical ring shaped structure that encircles DNA. The three domains (I, II, III) of each subunit have identical chain folding topologies and form an outside perimeter of a continuous antiparallel β sheet. The inside cavity is lined with 12 α helices. (B) Structure of a co-crystal of β with a primed DNA template (green). The side view reveals a tilted conformation of the β clamp on DNA with an angle of approximately 22°. (C) Model of the α subunit of E. coli Pol III bound to the β clamp and DNA (adapted with permission from Fig. 7 in 92).
