Abstract
AIM: To assess the role of hyoscine for polyp detection during colonoscopy.
METHODS: Studies (randomized controlled trials or RCTs) that compared the use of hyoscine vs no hyoscine or placebo for polyp detection during colonoscopy were included in our analysis. A search on multiple databases was performed in September 2013 with search terms being “hyoscine and colonoscopy”, “hyoscine and polyp”, “hyoscine and adenoma”, “antispasmotic and colonoscopy”, “antispasmotic and adenoma”, and “antispasmotic and polyp”. Jadad scoring was used to assess the quality of studies. The efficacy of hyoscine was analyzed using Mantel-Haenszel model for polyp and adenoma detection with odds ratio (OR). The I2 measure of inconsistency was used to assess heterogeneity (P < 0.05 or I2 > 50%). Statistical analysis was performed by RevMan 5.1. Funnel plots was used to assess publication bias.
RESULTS: The search of the electronic databases identified 283 articles. Of these articles, eight published RCTs performed at various locations in Europe, Asia, and Australia were included in our meta-analysis, seven published as manuscripts and one published as an abstract (n = 2307). All the studies included patients with a hyoscine and a no hyoscine/placebo group and were of adequate quality (Jadad score ≥ 2). Eight RCTs assessed the polyp detection rate (PDR) (n = 2307). The use of hyoscine demonstrated no statistically significant difference as compared to no hyoscine or placebo for PDR (OR = 1.06; 95%CI: 0.89-1.25; P = 0.51). Five RCTs assessed the adenoma detection rate (ADR) (n = 2015). The use of hyoscine demonstrated no statistically significant difference as compared to no hyoscine or placebo for ADR (OR = 1.12; 95%CI: 0.92-1.37; P = 0.25). Furthermore, the timing of hyoscine administration (given at cecal intubation or pre-procedure) demonstrated no differences in PDR compared to no hyoscine or placebo. Publication bias or heterogeneity was not observed for any of the outcomes.
CONCLUSION: Hyoscine use in patients undergoing colonoscopy does not appear to significantly increase the detection of polyps or adenomas.
Keywords: Hyoscine, Antispasmodic, Polyp detection, Colonoscopy
Core tip: Hyoscine is used in clinical practice to decrease spasms in the colon during colonoscopy in an effort to improve polyp or adenoma detection. However, this study shows that hyoscine given before the procedure or at time of cecal intubation does not improve polyp or adenoma detection.
INTRODUCTION
Colorectal cancer (CRC) is a common and devastating condition with higher incidence in developed countries/western world[1-4]. Screening programs have reduced the mortality related to CRC[5-7]. Most of these cancers arise from adenomatous polyps which can later progress into dysplasia and cancer; referred to as the adenoma-carcinoma sequence[8]. Colonoscopy is an important screening tool and has a large part in the reduction of CRC occurrence by removing these adenomatous polyps[9].
Adenoma detection rate (ADR) and polyp detection rate (PDR) are pivotal indicators for a quality colonoscopy and inversely related to the development of interval carcinoma[10,11]. Many polyps are missed with colonoscopy because of various factors, such as bowel preparation quality[12,13], polyp position[14,15], and colonic spasm[16,17]. Different antispasmodic agents including glucagon[18], dicyclomine[19], and atropine[20] have shown no significant benefit to facilitate colonoscopy.
Hyoscine butylbromide is a relatively safe antispasmodic anticholinergic agent which is commercially available in many forms (sublingual, injectable, and pills) and is frequently used to treat patients with functional bowel pain[21]. It blunts the response of colonic neurons to muscarinic and nicotinic stimulation which leads to inhibition of smooth muscle contraction in the colon[22]. It is associated with significantly less anticholinergic side effects due to not crossing the blood-brain barrier, making it a useful antispasmodic agent[23].
The use of hyoscine as premedication or at the time of cecal intubation during colonoscopy has shown conflicting results for detection of polyps[17,24-30]. Therefore, through study of randomized controlled trials (RCTs), hyoscine was compared to no hyoscine or placebo for polyp or adenoma detection during colonoscopy.
MATERIALS AND METHODS
Study selection
RCTs comparing hyoscine to no hyoscine or placebo on adults for polyp detection during colonoscopy were included. Criteria for exclusion was pediatric patients, non-randomized controlled trials, and abstract publications from other than the American College of Gastroenterology (ACG) and Digestive Disease Week (DDW) meetings or prior to 2003.
Data collection and extraction
Data was collected in multiple stages. First, a comprehensive search of PubMed/Medline, Embase, Cochrane databases, and CINAHL in September 2013 was conducted. Second, each selected article’s references were searched. Lastly, abstracts of DDW and ACG national meetings were searched from 2003-2013. The keywords used for the search included “hyoscine and colonoscopy”, “hyoscine and polyp”, “hyoscine and adenoma”, “antispasmotic and colonoscopy”, “antispasmotic and adenoma”, and “antispasmotic and polyp”. Standard forms were utilized for data extraction by three authors (IA, SA, and MLB) independently with any disagreements ruled on by a fourth author (AC) or mutual agreement. If data was incomplete or unclear, authors were contacted. Study quality was assessed by a Jadad score[31,32]. Jadad score ranges from 0 (poor quality) to 5 (excellent quality)[31]. It evaluates multiple study parameters related to randomization, blinding, and withdrawals. One point is deducted for each inappropriate criterion[31].
Statistical analysis
Pooled estimates of PDR and ADR were calculated for the effect of hyoscine or no hyoscine or placebo by odds ratio (OR) with Mantel-Haenszel (fixed effect) model given no heterogeneity identified.
Furthermore, a subgroup analysis was performed in similar fashion for the timing of the hyoscine administration, pre-procedure or during colonoscopy upon cecal intubation. I2 measure of inconsistency was used to assess heterogeneity (significant if P < 0.05 or I2 > 50%). Statistics performed by RevMan 5.1 (Review Manager Version 5.1. Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2012). Funnel plots, Egger’s regression intercept and Begg-Mazumdar rank correlation methods assessed publication bias.
RESULTS
Search of the electronic databases identified 283 articles (Figure 1). Of these articles, 8 published randomized controlled trials (RCTs) performed at various locations in Europe, Asia, and Australia were included in our meta-analysis, seven published as manuscripts[17,25-30] and one published as an abstract[24] (Table 1). All included patients with a hyoscine and a no hyoscine/placebo group and were of acceptable quality (≥ 2 on the Jadad scale) (Table 2).
Figure 1.
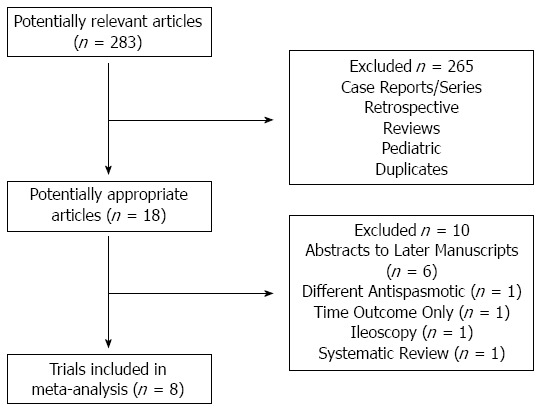
Selection of studies for inclusion in the meta-analysis.
Table 1.
Characteristics of studies included in meta-analysis
| Ref. | Study type | Blinding | Location | No. of patients | Hyoscine dose | Hyoscine route | Timing of administration |
| de Brouwer et al[26] 2012 | RCT | Double | Netherlands | 674 | 20 mg | IV | Cecal intubation |
| Byun et al[24] 2009 | RCT | Double | NR | 205 | 20 mg | IV | Cecal intubation |
| Lee et al[17] 2010 | RCT | Double | NR | 116 | 20 mg | IV | Cecal intubation |
| Kim et al[27] 2010 | RCT | Double | South Korea | 133 | 20 mg | IM | Premedication |
| Rondonotti et al[29] 2013 | RCT | Double | Italy | 402 | 20 mg | IV | Cecal intubation |
| Mui et al[28] 2004 | RCT | Yes | China | 120 | 40 mg | IV | Premedication |
| Saunders et al[30] 1996 | RCT | Yes | England | 56 | 20 mg | IV | Premedication |
| Corte et al[25] 2012 | RCT | Yes | Australia | 601 | 20 mg | IV | Cecal intubation |
RCT: Randomized controlled trial; NR: Not reported.
Table 2.
Quality assessment of the studies included in this meta-analysis using Jadad scale
| Ref. | Study design | Method of randomization | Double-blind | Method of double-blinding | Description of withdrawals | Total score2 |
| de Brouwer et al[26] 2012 | 1 | 1 | 1 | 0 | 1 | 4 |
| Byun et al[24] 20091 | 1 | 0 | 1 | 0 | 1 | 3 |
| Lee et al[17] 2010 | 1 | 1 | 1 | 0 | 1 | 4 |
| Kim et al[27] 2010 | 1 | 0 | 1 | 0 | 1 | 3 |
| Rondonotti et al[29] 2013 | 1 | 1 | 1 | 1 | 1 | 5 |
| Mui et al[28] 2004 | 1 | 1 | 1 | 1 | 1 | 5 |
| Saunders et al[30] 1996 | 1 | 1 | 1 | 1 | 1 | 5 |
| Corte et al[25] 2012 | 1 | 1 | 1 | 1 | 1 | 5 |
Abstract;
Jadad Score: 1-5, 5 is excellent and 1 is poor.
Polyp detection rate
Eight RCTs assessed the polyp detection rate (PDR) (n = 2307). The use of hyoscine demonstrated no statistically significant difference as compared to no hyoscine or placebo for PDR (502/1165, 43.1% vs 478/1142, 41.9%; OR = 1.06; 95%CI: 0.89-1.25; P = 0.51) (Figure 2). Statistically significant heterogeneity was not observed (I2 = 45%, P = 0.51).
Figure 2.
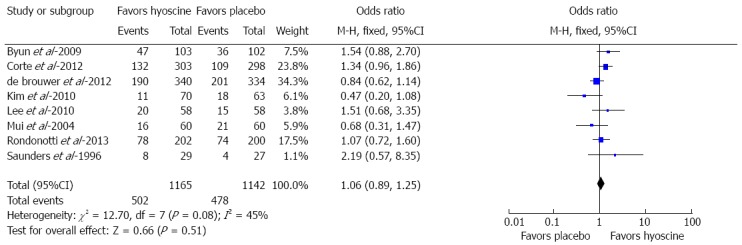
Forest plot showing no statistically significant difference in polyp detection rate between hyoscine and placebo group.
Adenoma detection rate
Five RCTs assessed the adenoma detection rate (ADR) (n = 2015). The use of hyoscine demonstrated no statistically significant difference as compared to no hyoscine or placebo for ADR (294/1018, 28.9% vs 266/997, 26.7%; OR = 1.12; 95%CI: 0.92-1.37; P = 0.25) (Figure 3). No heterogeneity was observed (I2 = 17%, P = 0.25).
Figure 3.
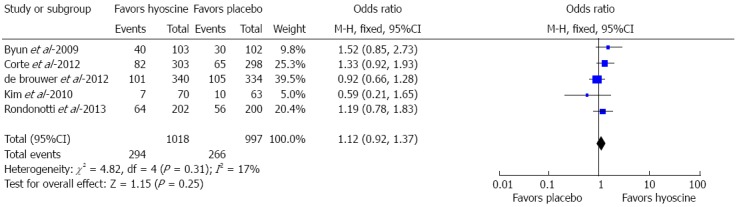
Forest plot showing no statistically significant difference in adenoma detection rate between hyoscine and placebo group.
Timing of hyoscine administration
On subgroup analysis, hyoscine administration given at cecal intubation showed no statistically significant difference in polyp (467/1006, 46.4% vs 435/996, 43.7%; OR = 1.12; 95%CI: 0.94-1.34; P = 0.22) or adenoma detection rate (287/948, 30.3% vs 256/934, 27.4%; OR = 1.15; 95%CI: 0.94-1.41; P = 0.17) as compared to no hyoscine or placebo. Furthermore, hyoscine administration pre-procedure showed no difference in PDR (35/159, 22% vs 43/150, 28.7%; OR = 0.71; 95%CI: 0.42-1.19; P = 0.19).
Publication bias
Publication bias was not observed as measured with funnel plot, Egger’s regression intercept method, or Begg-Mazumdar rank correlation method (Figure 4).
Figure 4.
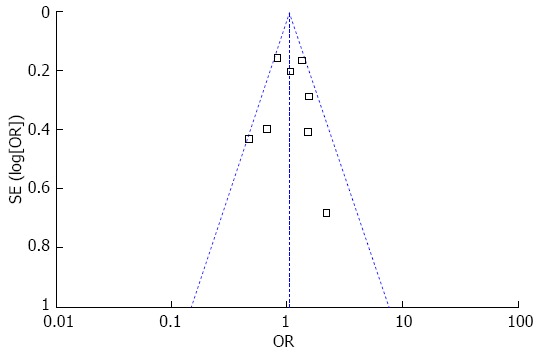
Funnel plot demonstrating no significant publication bias.
DISCUSSION
CRC is a preventable and curative condition if diagnosed early in the premalignant polyp stage. The quality of colonoscopy is very important as many polyps may be missed during screening colonoscopies which can lead to the development of interval carcinoma at a later stage[33]. Currently, ADR is considered one of the core parameters of a quality screening colonoscopy and better ADR can lead to decreased incidence of interval carcinoma[34].
Different medications including glucagon[18], dicyclomine[19], and atropine[20] have been tried to facilitate the colonoscopic exam with no significant improvement in results. Of all these agents, hyoscine has been evaluated extensively in RCTs with conflicting outcomes. Lee et al[17] found better PDR with the use of hyoscine with significant decrease in the colonic spasm. They did not notice any difference between the sites of polyps and suggested that hyoscine might be an option in patients with significant spasm[17]. Similarly, Corte et al[25] favored the use of hyoscine for screening and surveillance colonoscopy to aid in polyp detection. They did notice a difference in the withdrawal time and attributed that likely to the time spent on waiting for the spasm to resolve[25]. However, this was not designed primarily for ADR and considered PDR to be a surrogate marker for adenoma detection[25]. Despite these studies favoring the use of hyoscine to facilitate colonoscopy and polyp detection, other studies have showed contradictory results.
Byun et al[24] discovered no difference in polyp or ADR with more side effects with hyoscine use. Furthermore, procedure time and spasm score were not affected[24]. Similarly, de Brouwer et al[26] found no difference in PDR, ADR, or advanced lesions (> 1 cm) between the two groups. They did not appreciate any difference in withdrawal time between the two groups either but their study included gastroenterologists with more than 10-years’ experience[26]. More recently, Rondonotti et al[29] found similar results in a well-designed randomized controlled trial with no differences in ADR or advanced adenomas. Their findings rather opposed the use of hyoscine because of the lower detection of flat lesions with no difference in the procedure tolerance between the two groups[29]. Given that the results are conflicting and the high impact on performing a better colonoscopic exam, this meta-analysis was conducted.
An ideal antispasmodic agent should be able to decrease the total procedure time with better procedure tolerability, acceptable side effect profile, and should increase the polyp and adenoma detection rate. Although hyoscine is considered a relatively safe medication[35,36], Marshall et al[21] reported patients who developed sinus tachycardia with hyoscine. Similarly, Rondonotti et al[29] also reported an increased incidence of tachycardia in the hyoscine group. The finding of tachycardia in these studies lead to unblinding. Byun et al[24] also reported significant incidence of dry mouth with the use of hyoscine.
In this meta-analysis, hyoscine use did not show an increase in PDR or ADR during colonoscopy. No statistically significant differences between hyoscine vs no hyoscine or placebo in adenoma or polyp detection irrespective of timing of hyoscine administration (given at cecal intubation or pre-procedure). Recently, Cui et al[37] found similar results with a meta-analysis on this subject; however, it was limited to only five studies. Numerous strengths were apparent in this meta-analysis. First, a three-stage extensive article and abstract search was carried out. Second, various populations with a large number of patients were included. Third, the two main outcomes (adenoma and polyp detection) were evaluated in all included studies. Fourth, inclusion of high-quality positive and negative RCTs as evaluated by the Jadad score. Finally, publication bias was not observed. Despite the strengths, a few limitations are observed in our meta-analysis. First, timing of administration of hyoscine was different in these studies with some administering it as premedication while others gave it after cecal intubation (Table 1). Therefore, a subgroup analysis was performed to evaluate if timing made a difference and discovered that timing did not impact the results. Second, Mui et al[28] used a slightly higher dose (40 mg) compared to other studies. However, it this study was removed, the results were unchanged. Third, Kim et al[27] administered hyoscine intramuscularly which may have a slightly different bioavailability compared to IV form. Again, when this study was removed, no changes were observed in the outcomes.
In conclusion, hyoscine use during colonoscopy does not increase the polyp or adenoma detection rate. Therefore, hyoscine should not be routinely used in an effort to increase polyp detection during colonoscopy.
COMMENTS
Background
The main purpose of colonoscopy is to identify adenomatous polyps and colorectal cancers. Given potential of colonic spasms during colonoscopy, many agents have been studied to decrease the spasms in an effort to improve the adenomatous detection rate (ADR). One such agent is hyoscine.
Research frontiers
Hyoscine has been studied by multiple randomized controlled trials as an adjunct medication before or during colonoscopy to enhance ADR.
Innovations and breakthroughs
The authors found that hyoscine administered before or during colonoscopy does not appear to improve polyp detection rate (PDR) or ADR.
Applications
This information may limit the use of hyoscine before or during colonoscopy.
Terminology
Odds ratio: Statistical term for the odds an event did or did not occur. Heterogeneity: Test for uniformity in composition of studies included. Publication bias: Phenomenon where positive studies are more published more than negative studies, leading to possible misrepresentation of data in meta-analysis. PDR: Having one or more polyps identified during a colonoscopy. ADR: Having one or more adenomatous polyps identified on colonoscopy.
Peer review
The manuscript focussed on hyoscine for polyp detection during colonoscopy. The manuscript is well written.
Footnotes
P- Reviewer: Bustamante-Balen M, Deutsch JC, Gassler N S- Editor: Ji FF L- Editor: A E- Editor: Zhang DN
References
- 1.Jemal A, Tiwari RC, Murray T, Ghafoor A, Samuels A, Ward E, Feuer EJ, Thun MJ. Cancer statistics, 2004. CA Cancer J Clin. 2004;54:8–29. doi: 10.3322/canjclin.54.1.8. [DOI] [PubMed] [Google Scholar]
- 2.Cancer Research Campaign. Cancer Statistics: Large Bowel UK. London: CRC; 1999. Available from: http://www.cancerresearchuk.org/cancer-info/cancerstats/types/bowel/incidence/uk-bowel-cancer-incidence-statistics. [Google Scholar]
- 3.Jemal A, Siegel R, Ward E, Hao Y, Xu J, Thun MJ. Cancer statistics, 2009. CA Cancer J Clin. 2009;59:225–249. doi: 10.3322/caac.20006. [DOI] [PubMed] [Google Scholar]
- 4.Office of population Censuses and Surveys. Mortality statistics by cause: England and Wales, 1992. Series DH2, No. 20. London: HM Stationary Office; 1995. Available from: http: //www.ons.gov.uk/ons/rel/vsob1/mortality-statistics--cause--england-and-wales--series-dh2--discontinued-/no--20--1995/mortality-statistics--cause.pdf. [Google Scholar]
- 5.Mandel JS, Bond JH, Church TR, Snover DC, Bradley GM, Schuman LM, Ederer F. Reducing mortality from colorectal cancer by screening for fecal occult blood. Minnesota Colon Cancer Control Study. N Engl J Med. 1993;328:1365–1371. doi: 10.1056/NEJM199305133281901. [DOI] [PubMed] [Google Scholar]
- 6.Kronborg O, Fenger C, Olsen J, Jørgensen OD, Søndergaard O. Randomised study of screening for colorectal cancer with faecal-occult-blood test. Lancet. 1996;348:1467–1471. doi: 10.1016/S0140-6736(96)03430-7. [DOI] [PubMed] [Google Scholar]
- 7.Hardcastle JD, Chamberlain JO, Robinson MH, Moss SM, Amar SS, Balfour TW, James PD, Mangham CM. Randomised controlled trial of faecal-occult-blood screening for colorectal cancer. Lancet. 1996;348:1472–1477. doi: 10.1016/S0140-6736(96)03386-7. [DOI] [PubMed] [Google Scholar]
- 8.Fearon ER, Vogelstein B. A genetic model for colorectal tumorigenesis. Cell. 1990;61:759–767. doi: 10.1016/0092-8674(90)90186-i. [DOI] [PubMed] [Google Scholar]
- 9.Winawer SJ, Zauber AG, Ho MN, O’Brien MJ, Gottlieb LS, Sternberg SS, Waye JD, Schapiro M, Bond JH, Panish JF. Prevention of colorectal cancer by colonoscopic polypectomy. The National Polyp Study Workgroup. N Engl J Med. 1993;329:1977–1981. doi: 10.1056/NEJM199312303292701. [DOI] [PubMed] [Google Scholar]
- 10.Macken E, Moreels T, Vannoote J, Siersema PD, Van Cutsem E. Quality assurance in colonoscopy for colorectal cancer diagnosis. Eur J Surg Oncol. 2011;37:10–15. doi: 10.1016/j.ejso.2010.09.013. [DOI] [PubMed] [Google Scholar]
- 11.Kaminski MF, Regula J, Kraszewska E, Polkowski M, Wojciechowska U, Didkowska J, Zwierko M, Rupinski M, Nowacki MP, Butruk E. Quality indicators for colonoscopy and the risk of interval cancer. N Engl J Med. 2010;362:1795–1803. doi: 10.1056/NEJMoa0907667. [DOI] [PubMed] [Google Scholar]
- 12.Froehlich F, Wietlisbach V, Gonvers JJ, Burnand B, Vader JP. Impact of colonic cleansing on quality and diagnostic yield of colonoscopy: the European Panel of Appropriateness of Gastrointestinal Endoscopy European multicenter study. Gastrointest Endosc. 2005;61:378–384. doi: 10.1016/s0016-5107(04)02776-2. [DOI] [PubMed] [Google Scholar]
- 13.Harewood GC, Sharma VK, de Garmo P. Impact of colonoscopy preparation quality on detection of suspected colonic neoplasia. Gastrointest Endosc. 2003;58:76–79. doi: 10.1067/mge.2003.294. [DOI] [PubMed] [Google Scholar]
- 14.Rex DK, Cutler CS, Lemmel GT, Rahmani EY, Clark DW, Helper DJ, Lehman GA, Mark DG. Colonoscopic miss rates of adenomas determined by back-to-back colonoscopies. Gastroenterology. 1997;112:24–28. doi: 10.1016/s0016-5085(97)70214-2. [DOI] [PubMed] [Google Scholar]
- 15.Pickhardt PJ, Nugent PA, Mysliwiec PA, Choi JR, Schindler WR. Location of adenomas missed by optical colonoscopy. Ann Intern Med. 2004;141:352–359. doi: 10.7326/0003-4819-141-5-200409070-00009. [DOI] [PubMed] [Google Scholar]
- 16.Froehlich F. Colonoscopy: antispasmodics not only for premedication, but also during endoscope withdrawal? Gastrointest Endosc. 2000;51:379. doi: 10.1016/s0016-5107(00)70065-4. [DOI] [PubMed] [Google Scholar]
- 17.Lee JM, Cheon JH, Park JJ, Moon CM, Kim ES, Kim TI, Kim WH. Effects of Hyosine N-butyl bromide on the detection of polyps during colonoscopy. Hepatogastroenterology. 2010;57:90–94. [PubMed] [Google Scholar]
- 18.Norfleet RG. Premedication for colonoscopy: randomized, double-blind study of glucagon versus placebo. Gastrointest Endosc. 1978;24:164–165. doi: 10.1016/s0016-5107(78)73496-6. [DOI] [PubMed] [Google Scholar]
- 19.Bond JH, Chally CH, Blackwood WD. A controlled trial of premedication with dicyclomine hydrochloride (Bentyl) in colonoscopy. Gastrointest Endosc. 1974;21:61. doi: 10.1016/s0016-5107(74)73793-2. [DOI] [PubMed] [Google Scholar]
- 20.Waxman I, Mathews J, Gallagher J, Kidwell J, Collen MJ, Lewis JH, Cattau EL, al-Kawas FH, Fleischer DE, Benjamin SB. Limited benefit of atropine as premedication for colonoscopy. Gastrointest Endosc. 1991;37:329–331. doi: 10.1016/s0016-5107(91)70725-6. [DOI] [PubMed] [Google Scholar]
- 21.Marshall JB, Patel M, Mahajan RJ, Early DS, King PD, Banerjee B. Benefit of intravenous antispasmodic (hyoscyamine sulfate) as premedication for colonoscopy. Gastrointest Endosc. 1999;49:720–726. doi: 10.1016/s0016-5107(99)70289-0. [DOI] [PubMed] [Google Scholar]
- 22.Krueger D, Michel K, Allam S, Weiser T, Demir IE, Ceyhan GO, Zeller F, Schemann M. Effect of hyoscine butylbromide (Buscopan®) on cholinergic pathways in the human intestine. Neurogastroenterol Motil. 2013;25:e530–e539. doi: 10.1111/nmo.12156. [DOI] [PubMed] [Google Scholar]
- 23.Tytgat GN. Hyoscine butylbromide: a review of its use in the treatment of abdominal cramping and pain. Drugs. 2007;67:1343–1357. doi: 10.2165/00003495-200767090-00007. [DOI] [PubMed] [Google Scholar]
- 24.Byun TJ, Han DS, Ahn SB, Cho HS, Kim TY, Eun CS, Jeon YC, Sohn JH. Role of intravenous hyoscine N-butyl bromide at the time of colonoscopic withdrawal for polyp detection rates: A randomized, double-blinded, placebo-controlled trial. Gastrointest Endosc. 2009;69:AB229. [Google Scholar]
- 25.Corte C, Dahlenburg L, Selby W, Griffin S, Byrne C, Chua T, Kaffes A. Hyoscine butylbromide administered at the cecum increases polyp detection: a randomized double-blind placebo-controlled trial. Endoscopy. 2012;44:917–922. doi: 10.1055/s-0032-1310009. [DOI] [PubMed] [Google Scholar]
- 26.de Brouwer EJ, Arbouw ME, van der Zwet WC, van Herwaarden MA, Ledeboer M, Jansman FG, ter Borg F. Hyoscine N-butylbromide does not improve polyp detection during colonoscopy: a double-blind, randomized, placebo-controlled, clinical trial. Gastrointest Endosc. 2012;75:835–840. doi: 10.1016/j.gie.2011.12.010. [DOI] [PubMed] [Google Scholar]
- 27.Kim EO, Lee S, Kim DS, Lee CK, Lee TH, Chung I, Park S, Kim SJ. A clinical usefulness of premedication with Hyoscine N-butyl Bromide in colonoscopy. Korean J Gastrointest Endosc. 2010;41:10–15. [Google Scholar]
- 28.Mui LM, Ng EK, Chan KC, Ng CS, Yeung AC, Chan SK, Wong SK, Chung SC. Randomized, double-blinded, placebo-controlled trial of intravenously administered hyoscine N-butyl bromide in patients undergoing colonoscopy with patient-controlled sedation. Gastrointest Endosc. 2004;59:22–27. doi: 10.1016/s0016-5107(03)02377-0. [DOI] [PubMed] [Google Scholar]
- 29.Rondonotti E, Radaelli F, Paggi S, Amato A, Imperiali G, Terruzzi V, Mandelli G, Lenoci N, Terreni NL, Baccarin A, et al. Hyoscine N-butylbromide for adenoma detection during colonoscopy: a randomized, double-blind, placebo-controlled study. Dig Liver Dis. 2013;45:663–668. doi: 10.1016/j.dld.2013.01.029. [DOI] [PubMed] [Google Scholar]
- 30.Saunders BP, Williams CB. Premedication with intravenous antispasmodic speeds colonoscope insertion. Gastrointest Endosc. 1996;43:209–211. doi: 10.1016/s0016-5107(96)70317-6. [DOI] [PubMed] [Google Scholar]
- 31.Jadad AR, Moore RA, Carroll D, Jenkinson C, Reynolds DJ, Gavaghan DJ, McQuay HJ. Assessing the quality of reports of randomized clinical trials: is blinding necessary? Control Clin Trials. 1996;17:1–12. doi: 10.1016/0197-2456(95)00134-4. [DOI] [PubMed] [Google Scholar]
- 32.Jüni P, Altman DG, Egger M. Systematic reviews in health care: Assessing the quality of controlled clinical trials. BMJ. 2001;323:42–46. doi: 10.1136/bmj.323.7303.42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Brenner H, Chang-Claude J, Seiler CM, Hoffmeister M. Interval cancers after negative colonoscopy: population-based case-control study. Gut. 2012;61:1576–1582. doi: 10.1136/gutjnl-2011-301531. [DOI] [PubMed] [Google Scholar]
- 34.Pohl H, Robertson DJ. Colorectal cancers detected after colonoscopy frequently result from missed lesions. Clin Gastroenterol Hepatol. 2010;8:858–864. doi: 10.1016/j.cgh.2010.06.028. [DOI] [PubMed] [Google Scholar]
- 35.Tytgat GN. Hyoscine butylbromide - a review on its parenteral use in acute abdominal spasm and as an aid in abdominal diagnostic and therapeutic procedures. Curr Med Res Opin. 2008;24:3159–3173. doi: 10.1185/03007990802472700. [DOI] [PubMed] [Google Scholar]
- 36.Grainger SL, Smith SE. Dose-response relationships of intravenous hyoscine butylbromide and atropine sulphate on heart rate in healthy volunteers. Br J Clin Pharmacol. 1983;16:623–626. doi: 10.1111/j.1365-2125.1983.tb02231.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Cui PJ, Yao J, Han HZ, Zhao YJ, Yang J. Does hyoscine butylbromide really improve polyp detection during colonoscopy? A meta-analysis of randomized controlled trials. World J Gastroenterol. 2014;20:7034–7039. doi: 10.3748/wjg.v20.i22.7034. [DOI] [PMC free article] [PubMed] [Google Scholar]


