Figure 3. Transcriptional and posttranslational regulation of TLS polymerases.
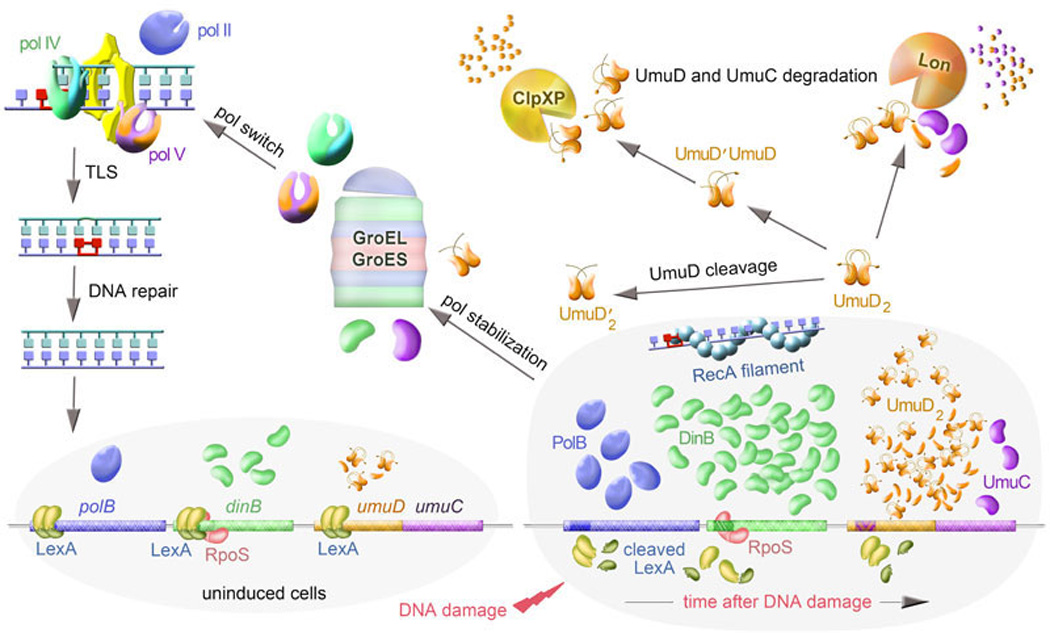
In uninduced cells, LexA (shown as yellow homodimer in which each monomer consists of an N-terminal DNA-binding domain and a C-terminal dimerization domain) represses expression of TLS polymerases (pol II represented in figures 3, 7, and 8 as a dark blue "right hand" shape and pol IV shown here and in figures 5–7 as a green "right hand" shape with a blue ““little finger” domain, as well as both, UmuC and UmuD subunits of pol V) to a different extend depending on Heterology Index (H.I.) of the LexA-binding site in the respective operator/promoter region of each polymerase. The H.I. also determines how soon after LexA cleavage expression of each gene is derepressed. DNA damage-induced formation of a RecA nucleoprotein filament on ssDNA mediates autocleavage of LexA and the derepression of genes in the LexA-regulon. It also mediates autocleavage of UmuD to UmuD'. Uncleaved and cleaved UmuD together with UmuC form different complexes of which UmuD'2C is the most stable. UmuD' is targeted for degradation by ClpXP through preferential formation of UmuD/UmuD' heterodimers. Both UmuC and UmuD are unstable and rapidly degraded by the Lon serine protease. However, UmuC is stabilized by the GroEL and GroES molecular chaperones. The groE gene products also stabilize pol IV. In addition to SOS induction, pol IV expression is also stimulated by RpoS (represented by a pink arched shape) in stressed cells. Upon repair of DNA damage, the disappearance of the RecA filament allows the newly synthesized LexA molecules to block transcription of all LexA-regulated genes and as a consequence, the intracellular concentrations of TLS polymerases return to their basal uninduced levels.
