Abstract
Hemangioblastoma (HB) of the central nervous system may occur sporadically or in association with von Hippel-Lindau (VHL) disease. Disseminated HB means malignant spread of the original primary HB without local recurrence at surgically resected site. It has been rarely reported previously, and rarer especially without VHL gene mutation. We report a case of disseminated HB without VHL disease. A 59-year-old man underwent a surgery for total removal of a cerebellar HB. From five years after the surgery, multiple dissemination of HB was identified intracranially and he subsequently underwent cyberknife radiosurgery. The lesions got smaller temporarily, but they soon grew larger. Nine years after the initial surgery for cerebellar HB, he showed severe back pain. His magnetic resonance image of spine revealed intradural extramedullary mass at T6-7 level. Complete surgical removal of the mass was performed and the pathological diagnosis was identical to the previous one. He had no evidence of VHL disease. And there was no recurrence of the tumor at the site of the original operation. The exact mechanism of dissemination is unknown, but the surgeon should be cautious of tumor cell spillage during surgery and prudently consider the decision to perform ventriculo-peritoneal shunt. In addition, continuous follow-up for recurrence or dissemination is necessary for patients even who underwent complete removal of cerebellar HB.
Keywords: Hemangioblastoma, Von Hippel-Lindau disease, Central nervous system
INTRODUCTION
Hemangioblastoma (HB) of the central nervous system (CNS) is a benign neoplasm which is classified as World Health Organization grade 1 CNS tumor. It usually occurs in the cerebellum, brainstem and spinal cord. It may occur sporadically or in association with von Hippel-Lindau (VHL) disease [1]. And it is generally considered to be a non-metastasizing tumor.
Von Hippel-Lindau disease is an autosomal dominant neoplasia syndrome caused by a germline mutation of the VHL gene which is mapped to chromosome 3p25. HBs in VHL disease are usually multiple, which is compared to sporadic HBs, and continue to arise over the course of a patient's lifetime [1,2].
The recurrence rate after surgery has been reported to be 15-27% [3], but diffuse spread and disseminated seeding are very rarely reported [4,5,6,7,8,9,10,11]. Disseminated HB has been first reported by Mohan et al. [8] in 1976, which is malignant spread of the original primary HB without local recurrence at surgically resected site. This means it is apparently different from typical recurrence. To our knowledge only 13 cases of disseminated hemangioblastoma have been reported previously [4,5,6,7,8,9,10,11]. Amongst them, 10 of the patients had no association with VHL disease. We add one case of disseminated HB without VHL disease and reviewed the reported cases previously.
CASE REPORT
In 2005, a 59-year-old male patient who suffered from occipital headache underwent complete surgical excision of a solitary cerebellar mass. Occipital transtentorial approach was done, and pathological diagnosis was confirmed to be hemangioblastoma (Fig. 1). He had no family history of VHL disease and no clinical stigmata to suggest the presence of VHL disease. VHL gene mutation was not detected in his peripheral blood sample. His symptom resolved after surgery, and the magnetic resonance image (MRI) of the brain showed no remnant lesion postoperatively. The patient was followed up with MRI, and no evidence of residual or recurrent lesion was observed until 2010.
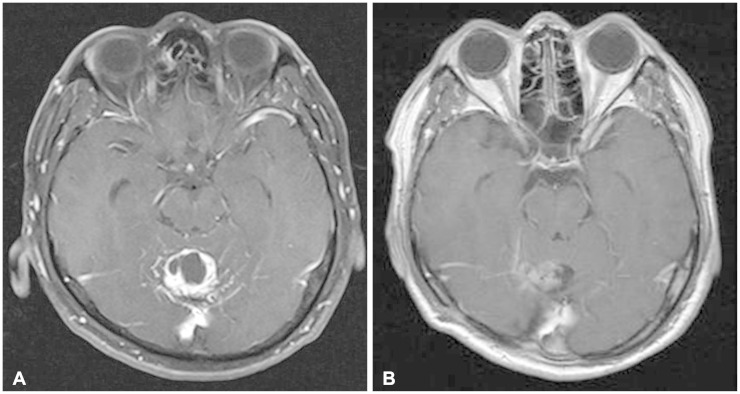
Fig. 1.
Pre and postoperative magnetic resonance images (MRIs). A: Preoperative gadolinium-enhanced T1-weighted MRI shows strongly enhancing mass involving the cerebellar vermis. B: Postoperative gadolinium-enhanced T1-weighted MRI. Tumor mass is completely removed and postoperative changes are observed.
In 2010, MRI of the brain showed a newly developed nodular mass at right medial edge of tentorium cerebelli suggesting dissemination of HB (Fig. 2). And in 2012, similar mass was observed at the posterior falx on the MRI (Fig. 2). There was no evidence of recurrence at the resected area and workup for VHL disease was also negative. He had no clinical symptom. He received cyberknife radiosurgery (total dose of 1,800 cGy/3 fx each) for each lesion in 2010 and 2012. After radiosurgery, the size of each lesion was reduced temporarily, but soon after the lesion at tentorium grew larger and other masses have newly developed around the pons and midbrain (Fig. 2).
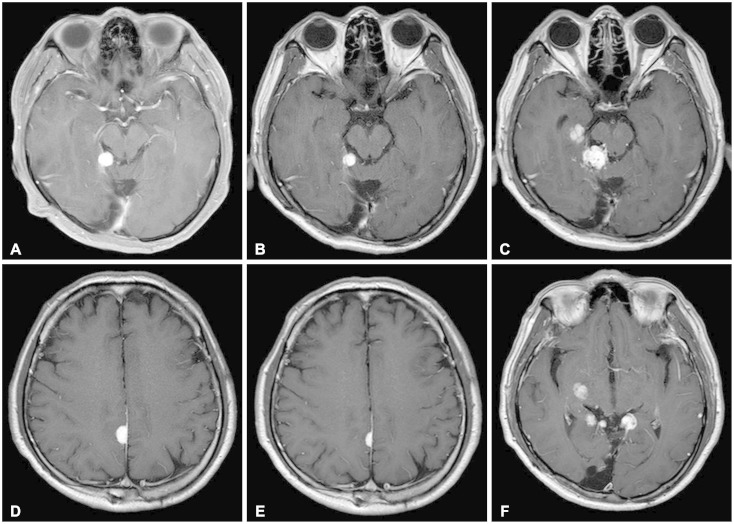
Fig. 2.
Gadolinium-enhanced T1-weighted magnetic resonance images of brain. A: Highly enhanced mass abutting the medial margin of the tentorium cerebelli was first noted in 2010. B: The size of tumor was reduced in 2012 (after cyberknife radiosurgery). C: But in 2014, previously noted mass showed enlargement, and newly developed mass was noted near ambient cistern. D: Similar mass was observed at posterior falx in 2012. E: It became smaller after cyberknife radiosurgery. F: Other disseminated lesions were observed near midbrain in 2014. A, B, and C: There was no evidence of recurrence at initially resected site.
Whole spine MRI revealed negative finding until 2012, but a intradural extramedullary mass was noted at T6-7 level in 2012 without any symptom (Fig. 3). In 2014, during follow-up of the thoracic lesion, he developed severe back pain at T9 dermatome, which correlated with the lesion. Spine MRI was done and it showed enlargement of the previously noted tumor mass at T6-7 level. Workup for VHL disease was done again, and the peripheral blood was analyzed for the presence of VHL gene mutations by direct sequencing method using ABI 3,730 sequencer. However, VHL gene mutation was not detected and there was no evidence of VHL disease. The patient underwent surgery for thoracic mass, and the tumor was completely removed (Fig. 4). The pathologic diagnosis of the tumor was HB, which is same as the cerebellar lesion resected previously (Fig. 5). The Ki-67 index was 10%. After surgery, his symptom was improved and until this case being reported, his Karnofsky Performance Scale score was 90 and remained symptom-free.
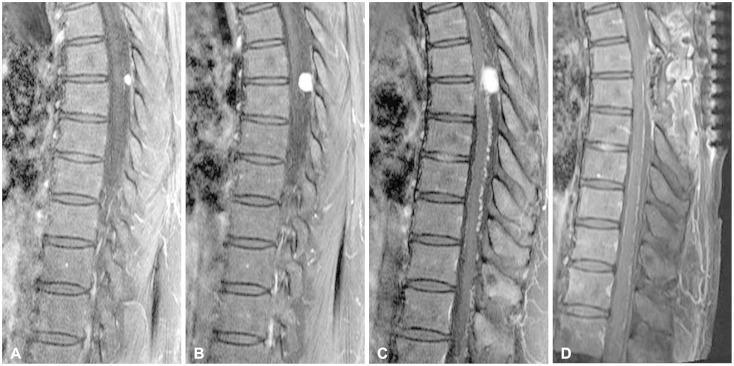
Fig. 3.
T1-weighted magnetic resonance images (MRIs) of thoracic spine obtained after gadolinium infusion. A: Small intradural extramedullary mass was observed at T6-7 level in 2012. B: Enlargement of the tumor size was noted in 2013. C: Preoperative image; MRI was taken when the patient showed neurological symptom in 2014, and the mass grew bigger. D: Postoperative image; the tumor was totally removed.
Fig. 4.
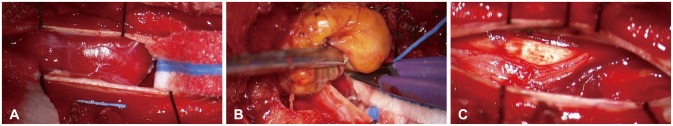
Intraoperative photographs at the T6-7 vertebral level. A: After opening the dura mater, we found highly vascularized tumor mass attached to the dorsal surface of spinal cord. B: Tumor was removed completely in en-bloc fashion after coagulating the surface of the tumor. C: Spinal cord was not injured, and the tumor was totally removed.
Fig. 5.
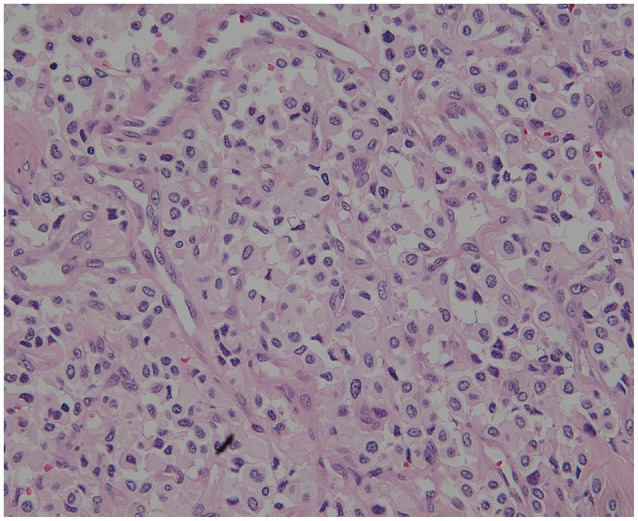
Histologic finding of tumor specimen stained with hematoxylin and eosin. Histopathological examination revealed endothelial, pericytic cells and stromal cells. The stromal cells was vacuolated and had larger nuclei with an eosinophilic foamy cytoplasm (×200).
DISCUSSION
Hemangioblastomas are benign neoplasm which originate exclusively from CNS tissues, and about 90% are found in cerebellum, brainstem and spinal cord [1]. HBs represent about 2% of all intracranial tumors and 7% to 12% of posterior fossa tumors [2]. And 2% to 10% of all primary spinal cord tumors are HBs. 60% to 75% of HBs occur sporadically and 25% to 40% occur in the context of the neoplasia syndrome, VHL disease [12].
Von Hippel-Lindau disease is an autosomal dominant neoplasia syndrome that has a prevalence of 1 in 39,000 live births [13]. It is characterized by the development of multiple visceral and CNS lesions. Blood samples can be tested for the VHL gene mutation, and the detection rate of VHL gene mutations in patients with a family history of VHL disease is nearly 100%. HB in VHL disease usually grows faster than sporadic HB. However, several recent studies suggest that VHL-related HBs and sporadic HBs grow similarly [1]. And there is no difference between sporadic and VHL-associated HBs with regard to recurrence or dissemination [1]. Though HB is thought to be curable by microsurgical resection and surgical outcomes are generally favorable, the recurrence was detected in 15% to 27% [3], which is higher than generally expected. It has a tendency to recur locally if resection is incomplete, but spreading elsewhere has been surely reported rare.
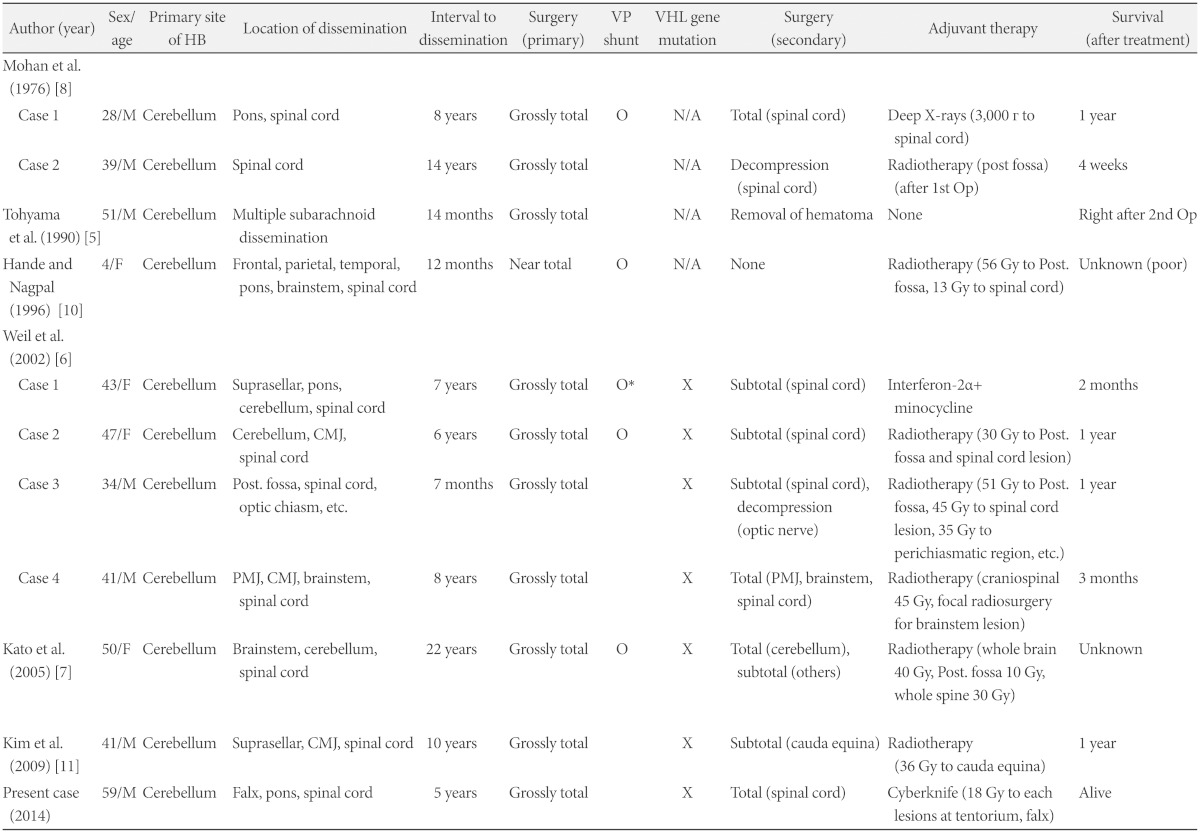
Dissemination of HB is an unusual type of recurrence, which was first reported by Mohan et al. [8] in 1976. Among 14 cases of disseminated hemangioblastoma reported previously including our case, 11 cases had no association with VHL disease (Table 1) [5,6,7,8,10,11]. The mean age of these 11 patients at presentation was 43.7 years (range, 4-59 years); four of the patients were female. On average, the time interval between initial operation and dissemination was 8.3 years (range, 7 months to 22 years). All of the patients underwent surgical excision of the primary lesion, and all of primary lesion was located in cerebellum. The primary tumors of ten cases were treated by grossly total removal, and only one case underwent near total removal. The location of dissemination was variable from supratentorial areas to spinal cord. Ventriculo-peritoneal shunt was performed in five cases. Among the 5 cases, one case was performed after dissemination, but the cytological study of the cerebrospinal fluid which was obtained intraoperatively revealed negative finding. In four cases which was reported before 2000, molecular genetic analysis of VHL gene mutation was not done. However, family history and clinical analysis showed negative for VHL disease in these four cases. In the other cases, VHL gene mutations were not detected in their peripheral blood samples. Every case underwent additional surgical removal of disseminated lesions, and in most cases adjuvant radiotherapy (range, 13-56 Gy) was done. However, long-term tumor control was not achieved. In one case, interferon-2α and minocycline was used as an adjuvant therapy to inhibit neo-angiogenesis, but it did not show notable tumor response. The outcomes after dissemination were very poor, and most patients died within 1 year. Most died by respiratory failure due to pontomedullary or cervical cord compression. However, in our case, the patient lived more than 5 years after dissemination and is still alive without any specific sequelae. This outcome is considered to be as a result of non-dissemination to the critical lesions as medulla oblongata or cervical spinal cord. In every reported case, biopsy specimens taken from the primary and secondary lesions were similar in histopathology.
Table 1.
Reported cases of disseminated hemagioblastomatosis of the central nervous system without von Hippel-Lindau disease
*after dissemination. CMJ: cervicomedullary junction, HB: hemangioblastoma, N/A: not available (genetic test not conducted), PMJ: pontomedullary junction, VP shunt: ventriculo-peritoneal shunt, X: gene mutation not detected
Hemangioblastoma is generally a radioresistent tumor. Long-term outcome of tumor control was mostly poor in many case reports which received radiotherapy as an adjuvant treatment postoperatively. This case was the first case which received radiosurgery as an adjuvant therapy for asymptomatic lesion in disseminated HB without VHL disease. The clinical outcome of our case was fine, but the regrowth of the tumor size was noted in the follow-up image studies. For the management of disseminated HB, all of currently available treatment did not show a significant effect on the progression of the disease even in sporadically arisen HB. It highlights the importance of continuous follow-up for patients presenting with HB. Even who underwent complete removal of HB needs close follow-up for early detection of recurrence or dissemination. The exact mechanism of dissemination is unknown, but as de novo development of disseminated HB without previous surgery has not been reported, surgical management of the primary lesion is strongly suggested as the source of dissemination. The surgeon should be cautious of tumor cell spillage during surgery and also prudently consider the decision to perform ventriculo-peritoneal shunt.
In conclusion, we report a rare case of disseminated HB of CNS without VHL disease. For the management of disseminated HB without VHL disease, there are currently no optimal treatments to prevent the progression of dissemination. The surgeon should be cautious of tumor cell spillage during surgical management of tumor and continuously follow-up the patients even who underwent complete removal of primary HB.
Footnotes
The authors have no financial conflicts of interest.
References
- 1.Choyke PL, Glenn GM, Walther MM, Patronas NJ, Linehan WM, Zbar B. von Hippel-Lindau disease: genetic, clinical, and imaging features. Radiology. 1995;194:629–642. doi: 10.1148/radiology.194.3.7862955. [DOI] [PubMed] [Google Scholar]
- 2.Neumann HP, Eggert HR, Weigel K, Friedburg H, Wiestler OD, Schollmeyer P. Hemangioblastomas of the central nervous system. A 10-year study with special reference to von Hippel-Lindau syndrome. J Neurosurg. 1989;70:24–30. doi: 10.3171/jns.1989.70.1.0024. [DOI] [PubMed] [Google Scholar]
- 3.Niemelä M, Lemeta S, Summanen P, et al. Long-term prognosis of haemangioblastoma of the CNS: impact of von Hippel-Lindau disease. Acta Neurochir (Wien) 1999;141:1147–1156. doi: 10.1007/s007010050412. [DOI] [PubMed] [Google Scholar]
- 4.Bakshi R, Mechtler LL, Patel MJ, Lindsay BD, Messinger S, Gibbons KJ. Spinal leptomeningeal hemangioblastomatosis in von Hippel-Lindau disease: magnetic resonance and pathological findings. J Neuroimaging. 1997;7:242–244. doi: 10.1111/jon199774242. [DOI] [PubMed] [Google Scholar]
- 5.Tohyama T, Kubo O, Kusano R, Miura N, Himuro H. [A case of hemangioblastoma with subarachnoid dissemination] No Shinkei Geka. 1990;18:83–88. [PubMed] [Google Scholar]
- 6.Weil RJ, Vortmeyer AO, Zhuang Z, et al. Clinical and molecular analysis of disseminated hemangioblastomatosis of the central nervous system in patients without von Hippel-Lindau disease. Report of four cases. J Neurosurg. 2002;96:775–787. doi: 10.3171/jns.2002.96.4.0775. [DOI] [PubMed] [Google Scholar]
- 7.Kato M, Ohe N, Okumura A, et al. Hemangioblastomatosis of the central nervous system without von Hippel-Lindau disease: a case report. J Neurooncol. 2005;72:267–270. doi: 10.1007/s11060-004-2244-7. [DOI] [PubMed] [Google Scholar]
- 8.Mohan J, Brownell B, Oppenheimer DR. Malignant spread of haemangioblastoma: report on two cases. J Neurol Neurosurg Psychiatry. 1976;39:515–525. doi: 10.1136/jnnp.39.6.515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Reyns N, Assaker R, Louis E, Lejeune JP. Leptomeningeal hemangioblastomatosis in a case of von Hippel-Lindau disease: case report. Neurosurgery. 2003;52:1212–1215. discussion 1215-6. [PubMed] [Google Scholar]
- 10.Hande AM, Nagpal RD. Cerebellar haemangioblastoma with extensive dissemination. Br J Neurosurg. 1996;10:507–511. doi: 10.1080/02688699647186. [DOI] [PubMed] [Google Scholar]
- 11.Kim HR, Suh YL, Kim JW, Lee JI. Disseminated hemangioblastomatosis of the central nervous system without von Hippel-Lindau disease: a case report. J Korean Med Sci. 2009;24:755–759. doi: 10.3346/jkms.2009.24.4.755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Singounas EG. Haemangioblastomas of the central nervous system. Acta Neurochir (Wien) 1978;44:107–113. doi: 10.1007/BF01401634. [DOI] [PubMed] [Google Scholar]
- 13.Conway JE, Chou D, Clatterbuck RE, Brem H, Long DM, Rigamonti D. Hemangioblastomas of the central nervous system in von Hippel-Lindau syndrome and sporadic disease. Neurosurgery. 2001;48:55–62. doi: 10.1097/00006123-200101000-00009. discussion 62-3. [DOI] [PubMed] [Google Scholar]