FIGURE 3.
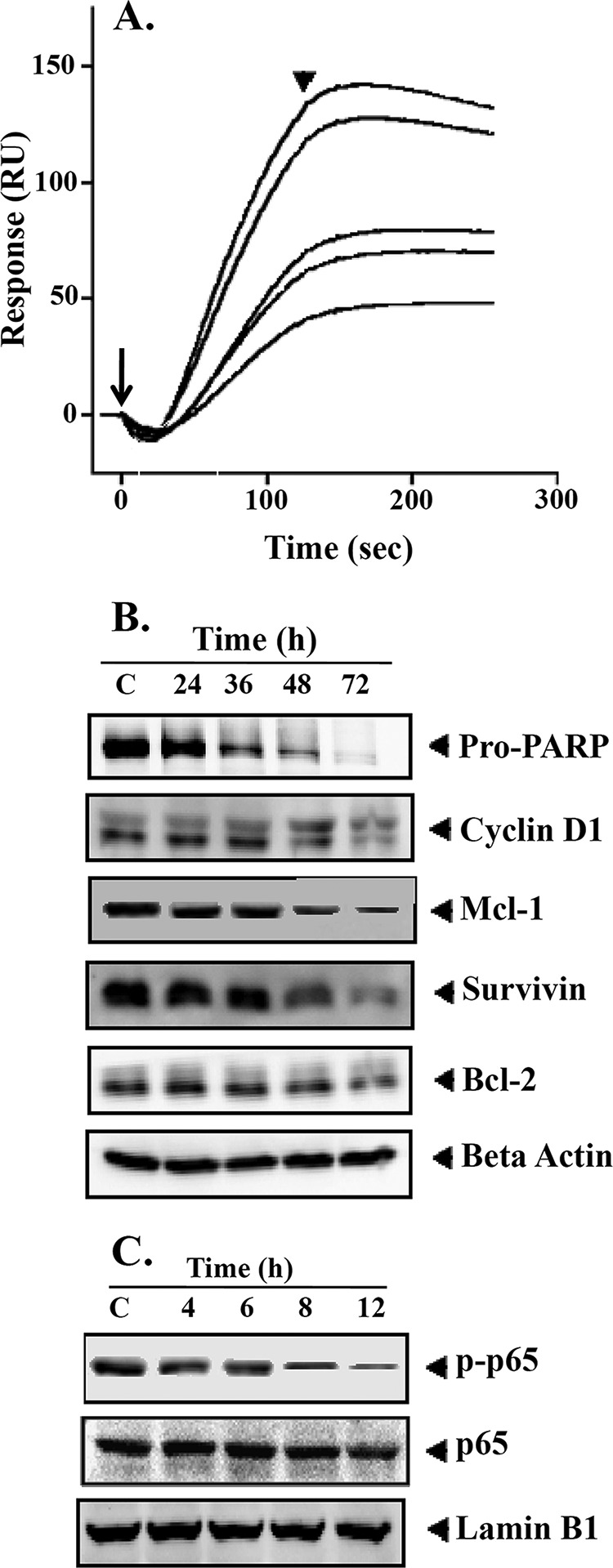
A, interaction of BIHC toward TNF-α. Various concentrations of BIHC (10–50 μm) were injected onto the surface of a TNF-α-immobilized sensor chip. Obtained sensorgrams were overlaid using the BIA evaluation software. RU, resonance units. Long and short arrows represent the beginning of the association and dissociation phases, respectively. B, BIHC suppresses NF-κB-regulated gene products involved in proliferation, survival, and angiogenesis. HepG2 cells (2 × 106/ml) were treated with 25 μm BIHC for the indicated time intervals, after which whole-cell extracts were prepared, 30 μg of proteins of those extracts was resolved on 10% SDS-PAGE, and membrane was sliced according to molecular weight and probed against PARP, cyclin D1, Mcl-1, survivin, and Bcl-2 antibodies. The same blots were stripped and reprobed with β-actin antibody to verify equal protein loading. The results of cyclin D1, Survivin, and Bcl-2 shown are representative of two independent experiments. C, HepG2 cells (2 × 106/ml) were treated with 25 μm BIHC for the indicated time intervals, after which whole-cell extracts were prepared and 30 μg of proteins of those extracts was resolved on 10% SDS-PAGE, and membrane was sliced according to molecular weight and probed against phospho-p65 antibody. The same blots were stripped and reprobed with p65 and lamin B1 antibodies.
