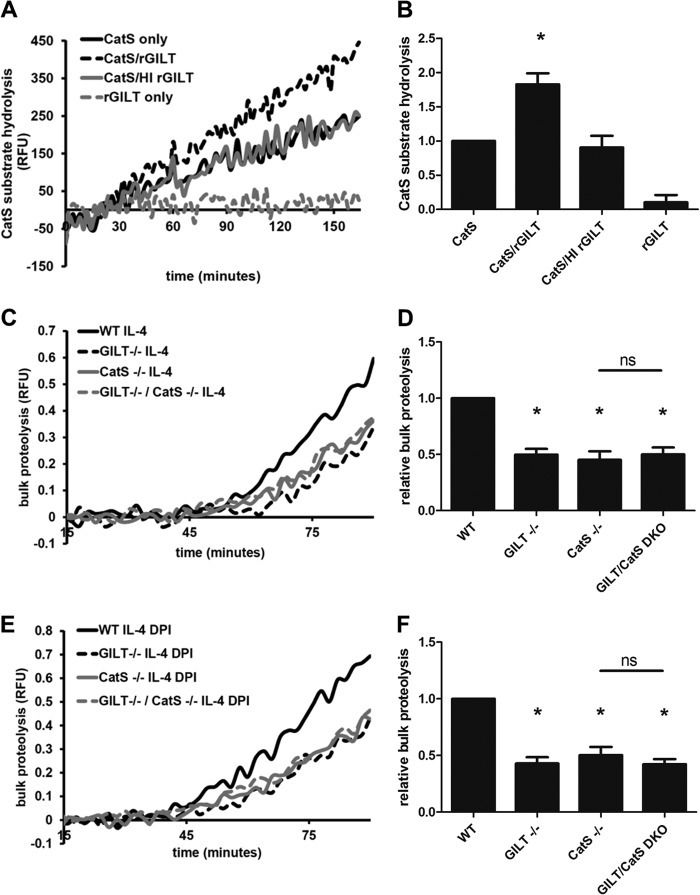
FIGURE 6.
GILT maintains cathepsin S activity in a reconstituted system and in the early phagosome of IL-4-activated BMMØs. A and B, purified cathepsin S (CatS) activity on the cathepsin S-specific substrate Ac-KQKLR-AMC in the presence/absence of rGILT in a reconstituted system. Where indicated, heat inactivation (HI) of rGILT was performed by subjecting the enzyme to 95 °C for 10 min before addition. *, p < 0.05 compared with CatS alone and CatS/HI rGILT samples. A, representative real-time traces. RFU, relative fluorescence units. B, averaged rates relative to CatS only controls from four independent experiments. Relative rates were calculated between 90 and 150 min. Error bars denote S.E. C and D, bulk phagosomal proteolysis as measured by hydrolysis of the DQ-albumin substrate conjugated to experimental particles in IL-4-activated BMMØs derived from WT, GILT−/−, CatS−/−, and GILT−/−/CatS−/− mice. E and F, bulk proteolysis as measured by hydrolysis of the DQ-albumin substrate conjugated to experimental particles in IL-4-activated BMMØs treated with the NOX2 inhibitor DPI (0.5 μm) 10 min before the addition and subsequent phagocytosis of experimental particles. C, and E, representative real-time traces. Relative fluorescence units values are proportional to the degree of substrate hydrolysis. D, and F, averaged rates relative to unactivated/untreated WT controls from five independent experiments. Relative rates were calculated between 40 and 60 min after particle internalization. Error bars denote S.E. *, p < 0.05 compared with WT control. ns, not significant.