FIGURE 6.
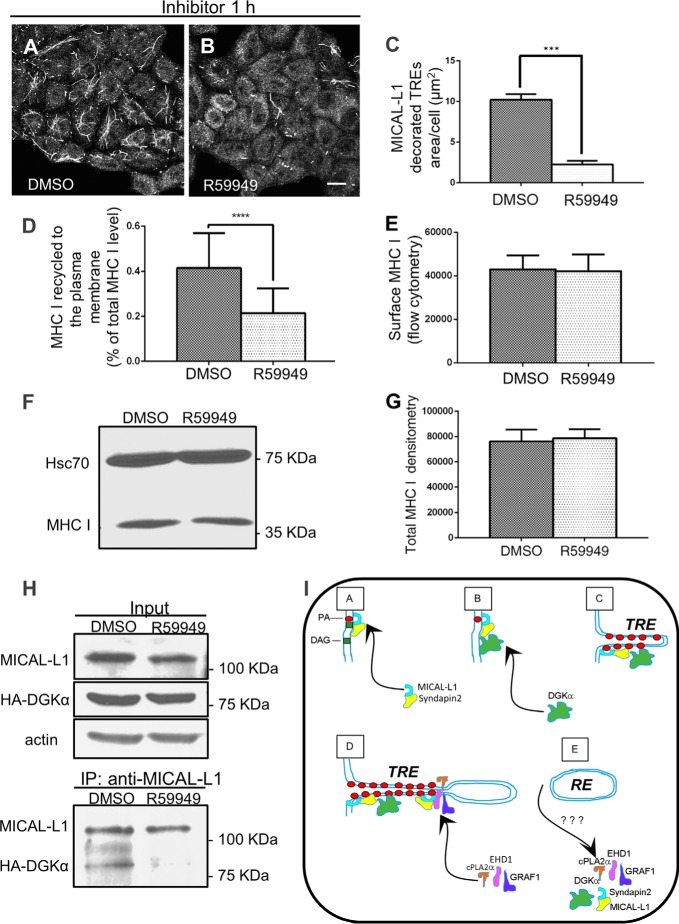
The biogenesis of tubular recycling endosomes requires the activity of DGKα. A and B, cells grown on coverslips were treated with either DMSO or 100 μm R59949 (DGK inhibitor) for 1 h at 37 °C, fixed-permeabilized, and then stained with anti-MICAL-L1. C, mean square area of TRE-localized endogenous MICAL-L1, taken from three independent experiments (from the experiments depicted in A and B), was measured with Image J and plotted with standard deviation, D, quantification of immunostained MHC I that re-appeared at the PM (underwent recycling). DMSO- and R59949-treated cells were pulsed with anti-MHC I antibody for 30 min at 37 °C, then chased for 3 h, followed by fixation. The cells were stained with Alexa-568 goat anti-mouse antibody in the absence of detergent. The total MHC I was measured in parallel with fixed cells incubated with anti-MHC I in the presence of detergent. The return of MHC I to the cell surface (following 3 h chase) was calculated as a portion of the total MHC I. 120 cells from three independent experiments were analyzed with Image J. E, surface level of MHC I for DMSO- and R59949-treated cells was measured by flow cytometry. F, total MHC I level of DMSO- or R59949-treated cells was measured by immunoblotting with anti-MHC I (HC10). Hsc70 was used as a loading control. G, graph depicts densitometry analysis of three independent experiments as in D. Shown is standard deviation. H, impaired PA production results in the loss of interaction between MICAL-L1 and DGKα. HA- DGKα transfected cells, growing on 100-mm dish were incubated with either DMSO or 100 μm R59949 for 1 h at 37 °C, and lysed (see input upper panel in H; anti-actin is a loading control). Lysates were co-immunoprecipitated with anti-MICAL-L1 antibodies (lower panel in H), and immunoblotted with anti-HA, and anti MICAL-L1. I, proposed model depicting the role of DGKα in TRE biogenesis. DGKα is recruited onto membranes through its interaction with a complex containing MICAL-L1. This leads to further generation of PA, and further recruitment of MICAL-L1, which serves as a membrane hub. Its association with the BAR-containing protein, Syndapin2, and the local enrichment of PA culminate in elongation of the membrane into a tubular endosome. Potential “vesiculators” such as EHD1, GRAF1, and cPLA2α are then recruited by MICAL-L1 and form a constriction site, ultimately leading to scission/budding of a transport vesicle. ***, p < 0.0005; ****, p < 0.0001. Bar, 10 μm.