FIGURE 3.
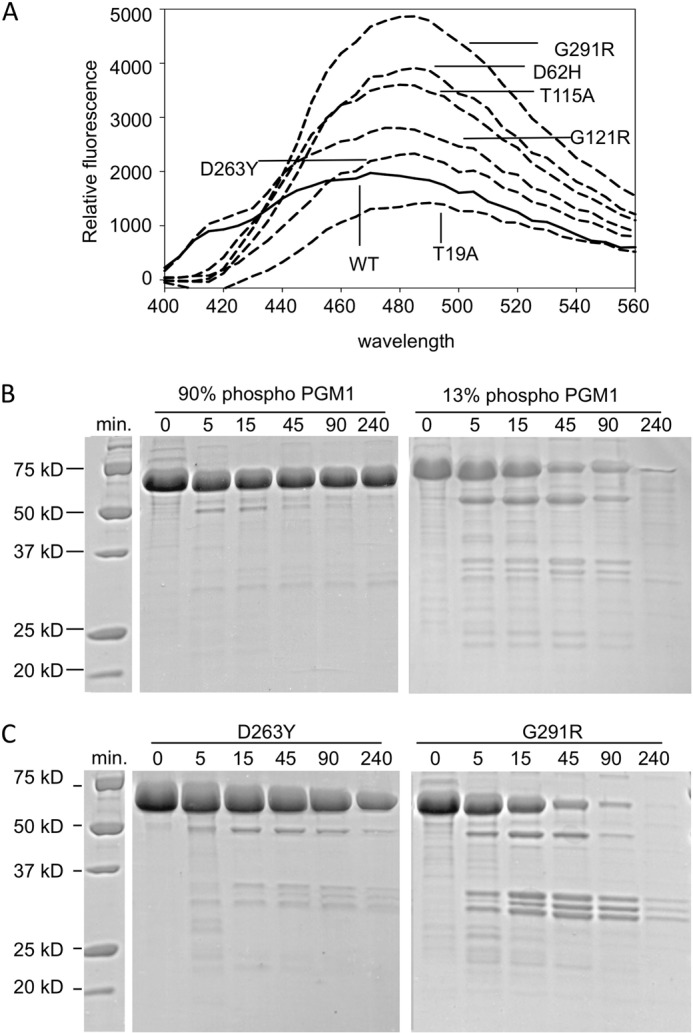
Effects of mutations on PGM1 flexibility and susceptibility to proteolysis. A, binding of ANS to WT PGM1 and the deficiency mutants (λex = 365 nm). SDS-PAGE showing limited proteolysis of (B) phosphorylated (90%) and dephosphorylated (13%) WT PGM1 (left and right panels, respectively), and (C) the D263Y (left) and G291R mutants (right). Length of digestion with proteinase K is given in minutes. Zero incubation time indicates untreated (control) samples. Note the increased susceptibility to proteolysis for both dephosphorylated PGM1 and the G121R mutant, which cannot be phosphorylated (Table 2).
