Figure 9.
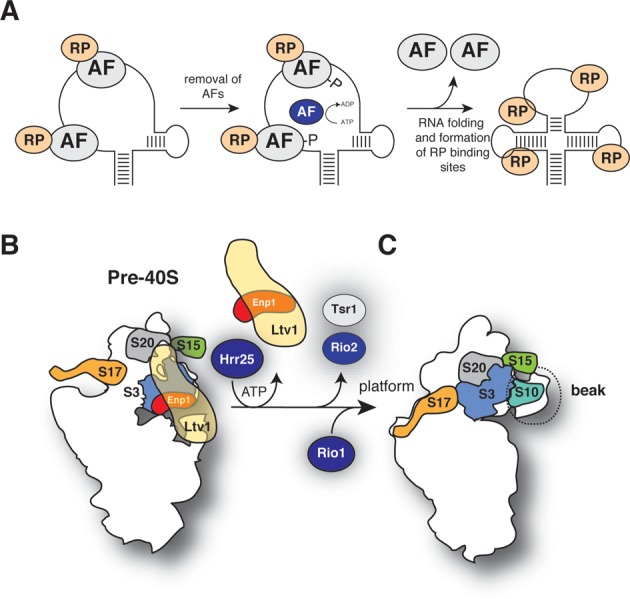
A model for structural rearrangements during late stages of 40S subunit maturation. (A) Proposed model for the function of ribosome assembly factors in 40S assembly. Binding of assembly factors (AF) to RNA is required to maintain a more flexible conformation. Their presence allows the assembly of ribosomal proteins (RP) but prevent that these RPs adopt their final conformation. At some point during the maturation pathway, the system receives a signal that certain ribosome assembly/RNA folding steps have been completed and AFs are no longer needed. Their release could be triggered through phosphorylation (such as Hrr25-dependent phosphorylation of Enp1 and Ltv1). This allows completion of RNA folding steps and binding of late RPs. (B and C) Model for rearrangements that take place in the head domain during late stages of 40S assembly. In early and middle pre-40S complexes, binding of Ltv1 and Enp1 to the head domain is required to maintain a more flexible conformation in the 3′ major domain. Rps3, Rps17, Rps20 and Rps17 assemble into pre-ribosomes but do not adopt their final conformation. Hrr25 phosphorylation of Ltv1 and Enp1 triggers their release, allowing late RNA folding steps and assembly of head domain r-proteins to be completed. We propose that Rio2 and Tsr1 are released shortly afterward (B) and Rio1 enters the assembly pathway. The exact function of Rio1, however, remains unclear.
