Figure 5.
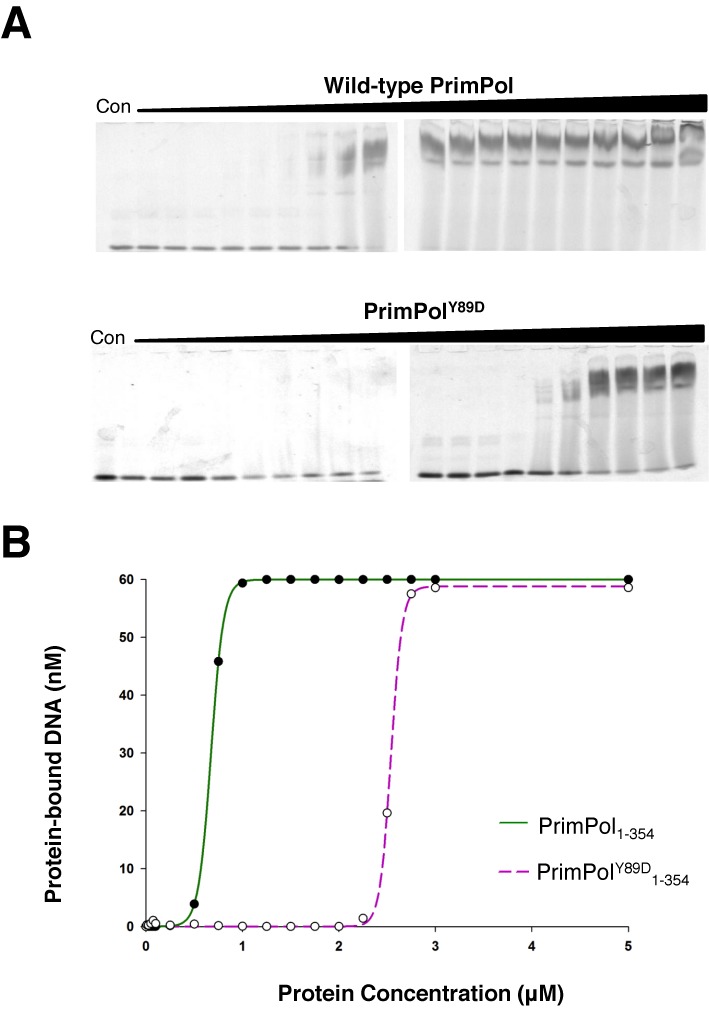
DNA binding efficiency of polymerase domains of wild-type and PrimPolY89D. (A) The polymerase domains (PrimPol1–354) for both wild-type and PrimPolY89D were used to eliminate the effects of binding from the zinc finger domains. Wild-type PrimPol domain bound to DNA at a much lower concentration than PrimPolY89D as determined by EMSAs. The concentrations used in both of these EMSAs were 0, 0.01, 0.025, 0.05, 0.075, 0.1, 0.25, 0.5, 0.75, 1, 1.25, 1.5, 1.75, 2, 2.25, 2.5, 2.75, 3, 5 and 10 μM. (B) These assays were subsequently quantified to determine KD(DNA) values for the polymerase domains of wild-type PrimPol (solid green line) and its Y89D variant (broken purple line). KD(DNA) of wild-type PrimPol was 0.69 μM, while the KD(DNA) of PrimPolY89D was 2.55 μM.
