Figure 1.
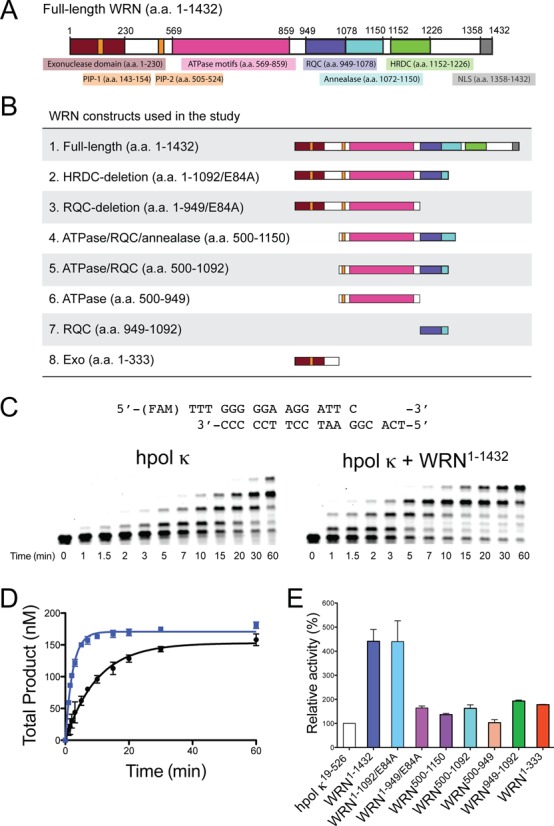
WRN stimulates hpol κ19-526 activity on undamaged DNA templates. (A) An overview of the full-length WRN protein showing domains with either structural or catalytic properties relevant to the current study. HRDC: Helicase and RNaseD C-terminal; NLS: nuclear localization signal; PIP: PCNA interacting peptide; RQC: RecQ C-terminal; the E84A mutation abrogates WRN exonuclease activity. (B) Schematic illustration of the eight WRN constructs utilized in the study. (C) DNA synthesis by hpol κ19-526 (2 nM) was monitored over time using a 13/18-mer primer-template DNA substrate (200 nM) in the absence of WRN and in the presence of full-length WRN1-1432 (100 nM). A schematic of the p/t-DNA substrate is shown above the gel results. (D) Total product formation [i.e. all of the product bands from panel (C)] was plotted as a function of time. The mean ± SEM is shown (n = 2). Product formation in each experiment was fit to a single-exponential equation [Equation (1)] to yield the following kinetic parameters: No WRN (black closed circles, •): A = 153 ± 4 nM, kobs = 0.099 ± 0.007 min−1; WRN1-1432 (blue closed squares,  ): A = 171 ± 2 nM, kobs = 0.43 ± 0.01 min−1. (E) The relative activity of hpol κ in the presence of different WRN constructs is shown. Polymerase extension assays with additional WRN constructs were performed as described in panel (C). The rate constants for the total product formed were 0.43 ± 0.02 (hpol κ + WRN1-1092/E84A), 0.16 ± 0.01 (hpol κ + WRN1-949/E84A), 0.13 ± 0.01 (hpol κ + WRN500-1150), 0.18 ± 0.01 (hpol κ + WRN500-1092), 0.094 ± 0.008 (hpol κ + WRN500-949), 0.18 ± 0.01 (hpol κ + WRN949-1092) and 0.19 ± 0.01 (hpol κ + WRN1-333) nM min−1. The values reported represent the mean ± SEM (n = 2). The relative activity of hpol κ was calculated by dividing the rate constant for primer extension in the presence of the WRN construct by the rate constant for primer extension by hpol κ alone then multiplying by 100.
): A = 171 ± 2 nM, kobs = 0.43 ± 0.01 min−1. (E) The relative activity of hpol κ in the presence of different WRN constructs is shown. Polymerase extension assays with additional WRN constructs were performed as described in panel (C). The rate constants for the total product formed were 0.43 ± 0.02 (hpol κ + WRN1-1092/E84A), 0.16 ± 0.01 (hpol κ + WRN1-949/E84A), 0.13 ± 0.01 (hpol κ + WRN500-1150), 0.18 ± 0.01 (hpol κ + WRN500-1092), 0.094 ± 0.008 (hpol κ + WRN500-949), 0.18 ± 0.01 (hpol κ + WRN949-1092) and 0.19 ± 0.01 (hpol κ + WRN1-333) nM min−1. The values reported represent the mean ± SEM (n = 2). The relative activity of hpol κ was calculated by dividing the rate constant for primer extension in the presence of the WRN construct by the rate constant for primer extension by hpol κ alone then multiplying by 100.
