Figure 2.
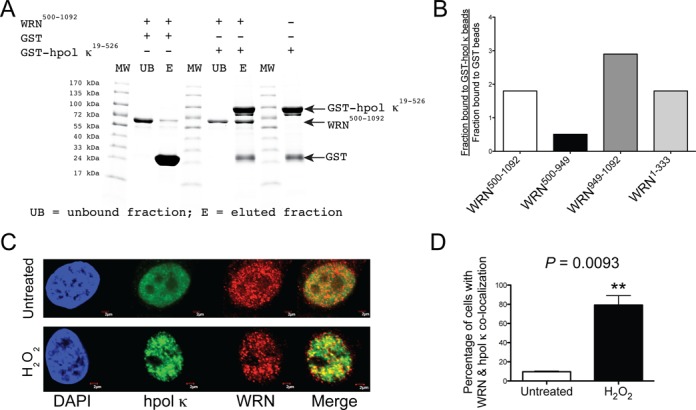
WRN and hpol κ physically interact and co-localize in cells following exposure to hydrogen peroxide. (A) Recombinant hpol κ possessing a glutathione transferase (GST) affinity tag (GST-hpol κ; 1200 pmol) was bound to glutathione (GSH)-coated beads and then incubated with WRN500-1092 (500 pmol). In a separate experiment, WRN500-1092 was incubated with GST-bound beads (i.e. no hpol κ present). The unbound fraction was removed from the beads; the beads were washed and bound proteins eluted by boiling in SDS-PAGE buffer. The unbound fraction and the eluted proteins were separated by SDS-PAGE and visualized with Coomassie blue. (B) The amount of WRN that bound GST-hpol κ-coated beads in a specific manner was quantified (see the Materials and Methods section for a description of the calculation). Specific binding to GST-hpol κ was observed for WRN RQC-containing constructs (a.a. 500-1092 and a.a. 949-1092) and for the WRN exo domain (a.a. 1-333). Specific binding was not observed for the WRN ATPase domain (a.a. 500-949). (C) WRN and hpol κ co-localize at nuclear foci following exposure to oxidative stress. HeLa cells grown on glass coverslips were either left untreated or were treated with H2O2 (0.5 mM, 4 h). The cells were fixed and immunostaining was performed with antibodies against hpol κ (green) and WRN (red). DAPI staining of the nucleus is also shown (blue). Co-localization of hpol κ and WRN is shown as yellow in the merged images. The scale bar represents 2 μm. (D) Quantification of WRN and hpol κ nuclear foci formation in untreated and H2O2-treated cells. A minimum of 50 cells were scored from three independent experiments. The mean (± SD) is shown. **P < 0.01.
