Abstract
The discovery of mammalian Toll-like receptors (TLRs), first identified in 1997 based on their homology with Drosophila Toll, greatly altered our understanding of how the innate immune system recognizes and responds to diverse microbial pathogens. TLRs are evolutionarily conserved type I transmembrane proteins expressed in both immune and non-immune cells and are typified by N-terminal leucine-rich repeats and a highly conserved C-terminal domain termed the Toll/interleukin (IL)-1 receptor (TIR) domain. Upon stimulation with their cognate ligands, TLR signaling elicits the production of cytokines, enzymes, and other inflammatory mediators that can impact several aspects of central nervous system (CNS) homeostasis and pathology. For example, TLR signaling plays a crucial role in initiating host defense responses during CNS microbial infection. Furthermore, TLRs are targets for many adjuvants which help shape pathogen-specific adaptive immune responses in addition to triggering innate immunity. Our knowledge of TLR expression and function in the CNS has greatly expanded over the last decade, with new data revealing that TLRs also impact non-infectious CNS diseases/injury. In particular, TLRs recognize a number of endogenous molecules liberated from damaged tissues and, as such, influence inflammatory responses during tissue injury and autoimmunity. Also, recent studies have implicated TLR involvement during neurogenesis and learning and memory in the absence of any underlying infectious etiology. Due to their presence and immune regulatory role within the brain, TLRs represent an attractive therapeutic target for numerous CNS disorders and infectious diseases. However, it is clear that TLRs can exert either beneficial or detrimental effects in the CNS, which likely depend on the context of tissue homeostasis or pathology. Therefore, any potential therapeutic manipulation of TLRs will require an understanding of the signals governing specific CNS disorders to achieve tailored therapy.
Keywords: Toll-like receptors, Central Nervous System, Bacterial Meningitis, Brain Abscess, Neurogenesis, Learning and Memory, Spinal Cord Injury, Alzheimer’s Disease
1. Overview of TLRs
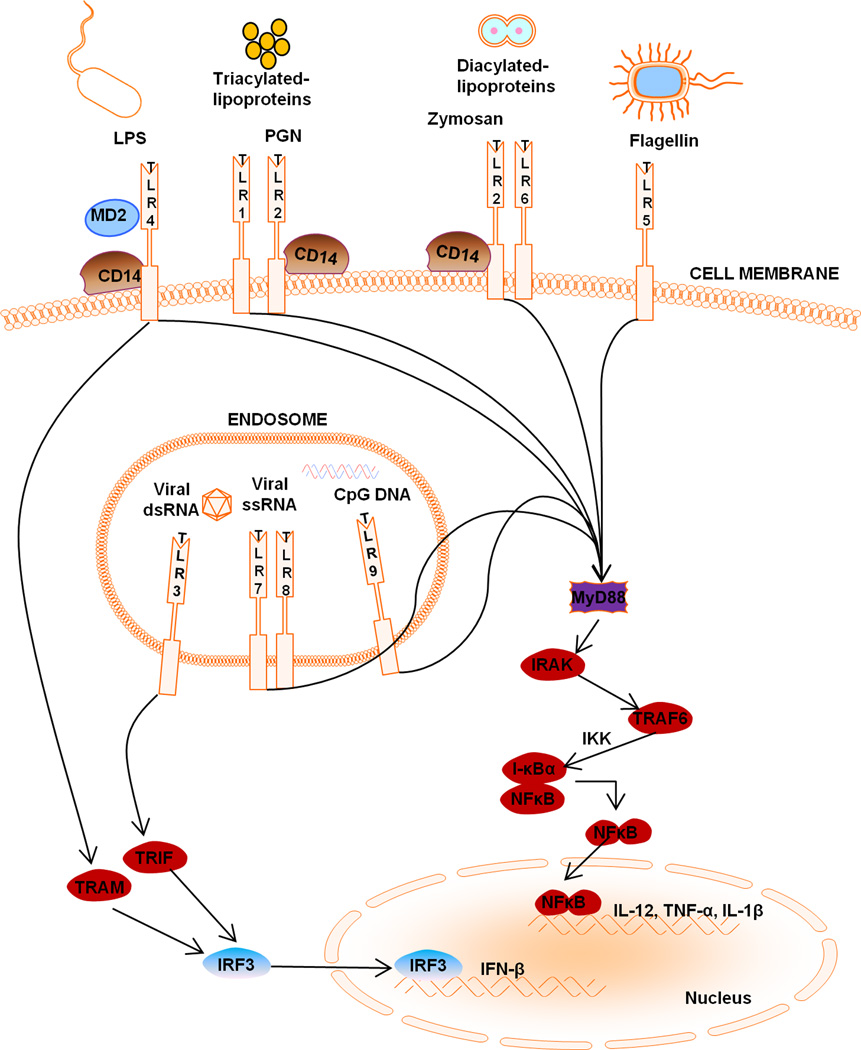
Innate immunity represents the first line of defense against invading microbes. Unlike the T and B cell receptors of the adaptive immune response, which can recognize an infinite antigenic repertoire due to random gene rearrangements, cells of the innate immune system rely on germ-line encoded receptors that are directed against broadly defined molecular motifs. These molecular determinants, termed pathogen-associated molecular patterns (PAMPs), are conserved among large classes of microorganisms and, as such, are unlikely to undergo mutation as they are essential for pathogen survival [1–4]. These PAMPs are sensed by pattern recognition receptors (PRRs), which comprise multiple receptor families located in both the extracellular and intracellular milieus. One such group of PRRs are TLRs, which are not only critical for eliciting innate immune responses to invading pathogens but are also important in initiating adaptive immunity [5, 6]. Specifically, TLR engagement on antigen presenting cells induces cytokine release and co-stimulatory molecule expression, priming these cells for subsequent activation and expansion of antigen-specific T cells [5–8] (Figure 1).
Figure 1. Toll-like receptor (TLR) localization and signaling.
TLR1, TLR2, TLR4, TLR5, and TLR6 are expressed at the cell surface for extracellular ligand recognition whereas TLR3, TLR7, and TLR9 are localized in the endosomal compartment for the recognition of pathogen nucleic acid motifs. All TLRs, with the exception of TLR3, recruit MyD88, while TLR1, TLR2, TLR4 and TLR6 recruit the additional adaptors CD14 and TIRAP, the latter of which links the TIR domain with MyD88. In the MyD88-dependent pathway, MyD88 recruits the IRAK family of proteins which leads to I-κB phosphorylation, resulting in the release and nuclear translocation of NF-κB, which influences the expression of numerous inflammatory genes. TLR3 ligands initiate the TRIF-dependent pathway, whereas TLR4 can signal via either MyD88-dependent or TRIF-dependent pathways requiring the additional linker adaptor TRAM, which links the TIR domain of TLR4 with TRIF. In the TRIF-dependent pathway, TRIF interacts with TRAF3 to activate IRF3 and IRF7 and initiate type I interferon production. Alternatively, TRIF can also bind to RIP1 and TRAF6 and activate NF-κB and MAPK for late-phase (i.e. 24 h) induction of inflammatory gene expression.
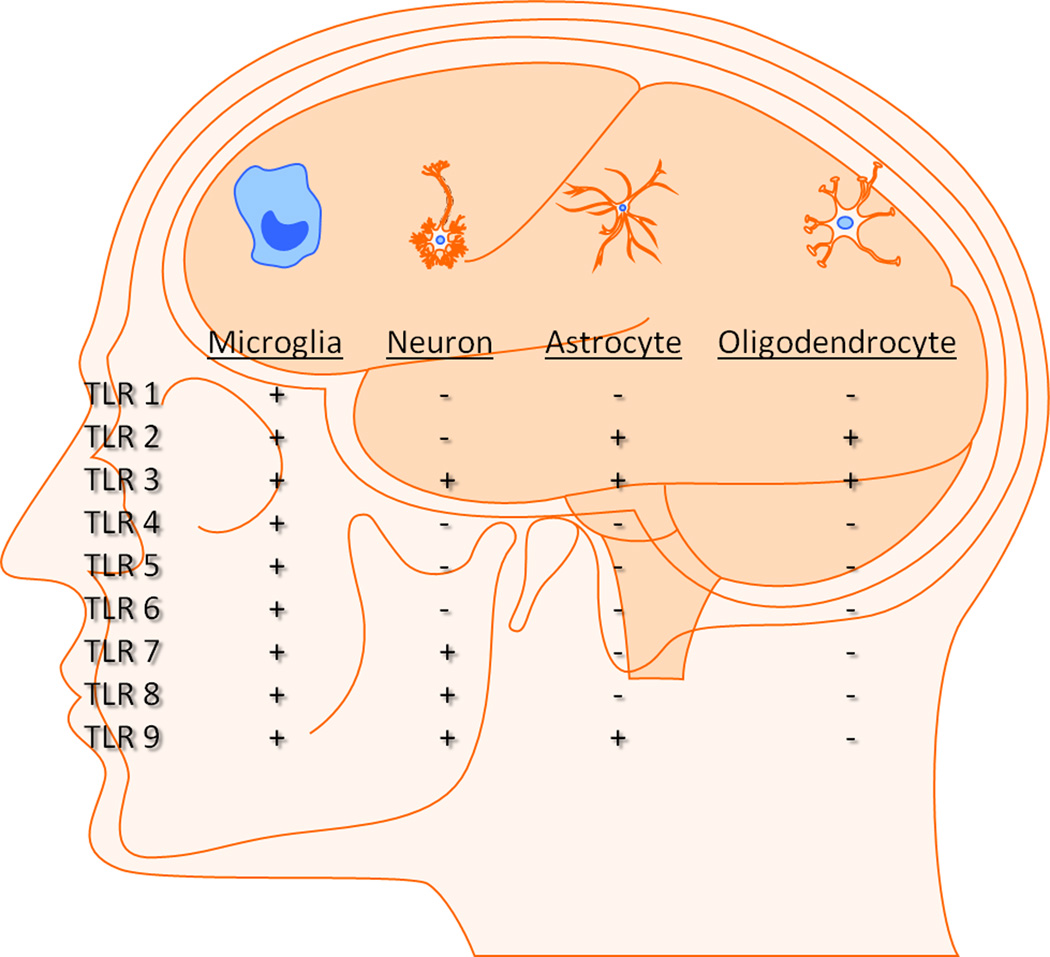
In addition to detecting molecular patterns associated with many microorganisms, TLRs have also been implicated in recognizing an array of endogenous molecules termed danger-associated molecular patterns (DAMPs) [3, 9–11]. In general, most DAMPs are sequestered from the immune response; however, during CNS infection and disease, self-antigens and/or danger signals may be liberated as a consequence of cell death, necrosis, or tissue remodeling. The expression of TLRs and related signaling proteins has been demonstrated in all major glial cell types, including microglia, astrocytes, and oligodendrocytes as well as a more limited repertoire in neurons [12–15] (Figure 2). Thus, TLR engagement may be elicited by a combination of PAMPs and/or DAMPs during inflammatory CNS disorders.
Figure 2. Expression of TLR family members in CNS cells.
Microglia express all TLRs identified to date, whereas astrocytes, oligodendrocytes and neurons express a more limited TLR repertoire in comparison.
The TLR family members, thirteen described in mice and eleven in humans, can be separated into two broad categories based on their subcellular localization patterns (Figure 1). To date, TLR2 and TLR4 are the best characterized and recognize the microbial motifs, peptidoglycan (PGN)/lipoproteins/dectin and lipopolysaccharide (LPS), respectively. These PAMPs are expressed within the pathogen cell wall and are accessible for TLR recognition once they come into contact with the cell surface [2]. TLR1 and TLR6 form heterodimers with TLR2 for the discrimination of triacylated from diacylated lipoproteins, respectively. TLR5 is responsible for sensing flagella of motile bacterial species and TLR11 recognizes pathogenic bacteria commonly associated with urinary tract infections such as uropathogenic E. coli as well as a profilin-like protein from the parasite Toxoplasma gondii [16–18]. TLR3, TLR7/8, and TLR9 recognize intracellular pathogen-derived nucleic acid motifs. Specifically, viral infections lead to the generation of dsRNA or ssRNA intermediates depending on the pathogen, which can trigger TLR3 and TLR7/8, respectively. In addition, TLR9 recognizes nonmethylated CpG motifs of bacterial and viral DNA [3, 19, 20] (Table 1). Although these sequences also occur in mammalian DNA, they are typically methylated and thus, do not trigger TLR9-mediated signaling. Following infection, it is likely that these intracellular TLRs serve to amplify responses initially triggered by extracellular TLRs to ensure effective pathogen clearance. In the case of neurodegenerative diseases without evidence of an infectious etiology, pathologic engagement of TLRs by DAMPs may contribute to exacerbated immune responses and enhanced neuropathology. Alternatively, some studies suggest a neuroprotective role for TLR signaling, indicating that the context and intensity of TLR engagement may dictate whether TLRs exert beneficial vs. detrimental properties during CNS disorders.
Table 1.
Major TLR agonists
| TLR | Agonists |
|---|---|
| 1/2 | Triacylaled lipoproteins and peptidoglycan |
| 2 | Lipoproteins and peptidoglycan |
| 3 | Double-stranded (ds) RNA |
| 4 | Lipopolysaccharide (LPS) |
| 5 | Flageellin |
| 6/2 | Diacvlated lipoproteins and Zymosan |
| 7 | Single-stranded (ss) RNA |
| 8 | Single-stranded (ss) RNA |
| 9 | Nonmethylated cytosine-guanosine containing oligonucleotides (CpG)-DNA |
| 11 | T. gondii profiln, E. coli |
Depending on the particular TLR, receptor engagement can culminate in the induction of nuclear factor-kappa B (NF-κB), mitogen-activated protein kinases (MAPK), and/or interferon-regulatory factor (IRF) signaling pathways, which regulate the expression of a wide array of genes involved in inflammatory responses (Figure 2). The majority of TLRs utilize the central adaptor molecule Myeloid differentiation primary response gene 88 (MyD88), with the exception of TLR3, to bridge the receptor to downstream signaling intermediates [2, 21]. TLR activation results in MyD88 recruitment, which is associated with the serine/threonine kinase interleukin-1 receptor-associated kinase (IRAK) (Figure 1). Subsequently, IRAK interacts with TNF receptor-associated factor (TRAF) adaptor protein TRAF6, and provides a link to NF-κB-inducing kinase (NIK). NIK then phosphorylates I-κB kinase (IKK), leading to I-κB phosphorylation. I-κB phosphorylation targets the protein for ubiquitination and proteasome-mediated degradation, resulting in the release and nuclear translocation of NF-κB whereupon it can influence the expression of numerous immune response genes. In addition to MyD88, alternative adaptor molecules have been identified that initiate responses emanating from TLR3 and TLR4 [2]. These MyD88-independent adaptors include TIR domain-containing adaptor inducing interferon-β (TRIF) and TRIF-related adaptor molecule (TRAM), which are pivotal for the expression of IFN-inducible genes following TLR4 activation [2, 22, 23]. TRIF is also required for TLR3-mediated signaling in response to dsRNA and is responsible for the induction of type I interferons (i.e., IFN-α and IFN-β) that are a hallmark of the host innate immune response to viral infection (Figure 1).
Following receptor engagement, TLRs initiate signaling cascades that lead to the production of a wide array of proinflammatory mediators, including reactive oxygen/nitrogen intermediates, cytokines, and chemokines [24–26]. In general, the net effect of TLR signaling during CNS infection is to facilitate innate immune activation as well as to recruit and activate various immune cell populations. However, it is important to acknowledge that the outcomes of TLR signaling in response to endogenous “danger” signals may elicit a different secretory response tailored to the type of insult. In this review, we will discuss TLRs in CNS health and disease, focusing on the mechanisms and therapeutic potential of TLRs in the brain.
2. TLR Expression and Function in CNS Intrinsic Cell Types
It is probable that multiple PRRs are employed to elicit a maximal response to infectious agents. For instance, intact bacteria likely engage TLRs in addition to phagocytic receptors on microglia that facilitate pathogen clearance. The potent proinflammatory response that ensues after TLR ligation recruits professional phagocytes from the peripheral circulation that are essential for the genesis of innate immune responses to infection. Conversely, it is possible that PAMPs released from dead bacteria, as well as DAMPs liberated during tissue injury result in the false detection of an active infection by innate immune cells through continual TLR ligation, even though no viable infectious agents remain. Thus, therapeutic strategies designed to halt this cyclical activation cascade would be expected to circumvent prolonged insult to normal parenchyma associated with infected and damaged tissues.
2.1 Microglia
Microglia are the resident innate immune cell population in the CNS parenchyma, capable of recognizing a wide array of infections agents [27]. Microglia sense pathogens through a cadre of various PRRs, including TLRs, Nod-like receptors (NLRs) and scavenger receptors. The focus of this review is to summarize the current knowledge base regarding TLR expression and function during CNS disease and homeostasis. The reader is referred to other excellent reviews covering alternative PRR families [28–31].
Microglia express all known members of the TLR family identified to date. Microglia constitutively express TLR2 [32–39] and are capable of recognizing numerous TLR2 ligands including lipoproteins and the synthetic lipoprotein analog tripalmitoyl-S-glyceryl-cysteine (Pam3Cys), as well as LTA, and PGN [32, 35, 40–42]. Our group was the first to report that TLR2 plays a functional role in PGN recognition using primary microglia isolated from TLR2 knock-out (KO) mice [37]. Interestingly, although TLR2 is essential for PGN recognition in primary microglia we found that microglial responses to intact Gram-positive bacteria were largely TLR2-independent, with the exception of the IL-12 family members, which were reproducibly elevated in TLR2 KO microglia [37]. These results suggest that alternative receptors are involved in signaling proinflammatory mediator production in response to intact S. aureus. Indeed, recent studies from our laboratory have identified that intact bacteria trigger TLR9 activation, which in the absence of TLR2 signaling, leads to the unchecked production of IL-12 family member cytokines (Holley and Kielian, submitted).
Microglia express TLR3 [35, 38] and respond to the synthetic TLR3 agonist poly-inosine:cytosine [poly(I:C)] and Theiler’s murine encephalomyelitis virus (TMEV) with elevated immune receptor expression as well as the production of select cytokines including IFN-β, IL-1β, and IL-6 [35]. Interestingly, TLR3 expression does not appear to be regulated by poly(I:C) in microglia, which differs from other TLRs where receptor levels are augmented following exposure to their natural ligands [35, 37]. LPS has long been recognized as a potent stimulus for microglial activation; therefore, it follows that numerous studies have reported TLR4 expression on microglia [33, 35, 38, 42–46]. The use of microglia from TLR4-deficient mice has allowed for the direct demonstration that this receptor is responsible, in part, for microglial activation in response LPS [47, 48]. Several reports have demonstrated that microglia express TLR9 and respond to CpG DNA with the robust production of numerous proinflammatory mediators [35, 49–52]. Related to the ability of CpG DNA to induce proinflammatory mediator release, CpG ODN-stimulated microglia were found to induce neuronal cell death in a neuron-microglia co-culture paradigm [51]. This microglial-mediated neuronal toxicity following CpG ODN treatment is reminiscent of what is observed following stimulation with the TLR4 agonist LPS [53], suggesting, in part, the conservation of microglial responses to diverse PAMPs and a link between TLRs and neurodegeneration.
TLR7 and TLR8 are highly homologous and important for immune responses elicited by GU-rich single stranded RNA as well as synthetic chemicals including imidazoquinoline compounds (i.e. imiquimod and resiquimod) and guanosine analogs [54–56]. Both TLR7 and TLR8 are expressed in microglia [35, 38] and recent studies have demonstrated the importance of TLR7 signaling in regulating microglial proinflammatory mediator expression and cross-talk with TLR9 [57].
In contrast to TLR2, TLR4, TLR7/8, and TLR9, fewer studies have been performed characterizing the functional activity of alternative TLRs expressed by microglia. Although microglia also express TLR1, TLR5, TLR6, TLR10, and TLR11, to our knowledge, little is known regarding the functional significance of these receptors by studying KO microglia. TLR10 is an orphan member of the TLR family and has recently been described to form homodimers as well as heterodimerize with TLR1 and TLR2 [58]. The expression of TLR10 appears to be rather restricted, with only B cells and specialized dendritic cell subsets demonstrating expression [58]. Mouse TLR11 is involved in the recognition of uropathogenic bacteria (such as E. coli) as well as a profilin-like molecule of Toxoplasma gondii, whereas the human receptor is non-functional because of the presence of a stop codon [16–18]. Although these TLRs reportedly have limited and unique expression patterns, a recent study has demonstrated their presence in the normal CNS and following parasitic infection [59].
Although we are beginning to understand the functional consequences of TLR signaling in microglia, there is relatively little information available addressing how microglia are able to discriminate between TLR signals and tailor their responses to be appropriate in intensity and duration for a given inciting stimulus. For example, all TLRs elicit NF-κB activation; however, the types of responses required for bacterial killing versus β-amyloid recognition are expected to differ greatly. In addition, mechanisms must exist to enable microglia to discriminate between extracellular versus intracellular bacteria, even though many common TLRs are triggered in both scenarios. The concept of tailoring TLR responses appropriate for the stimulus context is an area where additional studies are needed.
2.2 Astrocytes
Astrocytes are the major glial cell type in the CNS and can also participate in inflammatory responses. Astrocytes express a more limited TLR repertoire (Figure 2), which likely stems from the fact that they are not classical immune cells based on their neuroectodermal origin, but can contribute to inflammation if necessary. Indeed, astrocytes are recognized as a prevalent source of chemokines following CNS insult/infection [60].
Several studies have demonstrated TLR2 expression in primary mouse astrocytes that was augmented following treatment with various PAMPs [61–63]. Our laboratory was the first to demonstrate that TLR2 is pivotal for astrocyte recognition of both intact S. aureus as well as PGN [63]. A recent study has demonstrated that prior exposure of astrocytes to proinflammatory stimuli leads to augmented TLR2 expression and subsequent hyper-reactivity following treatment with TLR2 ligands [64]. These findings have implications for ongoing inflammatory responses and may have identified a pathological feedback loop that perpetuates CNS inflammation. Astrocytes also express TLR3 and are responsive to the TLR3 agonist poly(I:C) as evident by the production of several proinflammatory mediators [15, 38, 62, 65]. Gene profiling of activated astrocytes demonstrated that TLR3 signaling induced a comprehensive neuroprotective response, typified by the expression of numerous neuroprotective mediators and several other molecules that regulate cellular growth, differentiation, and migration rather than a polarized proinflammatory reaction [66].
In contrast to microglia, TLR4 expression in astrocytes appears more controversial. Specifically, several groups have been unable to demonstrate astrocytic TLR4 expression in vitro [15] or in vivo [43, 46, 53]; however, others have been able to detect low, constitutive expression of TLR4 in astrocytes that is increased upon cell activation [38, 61, 62, 67]. The reasons for these discrepancies are not clear but could be the result of species-specificity or differences between in vitro versus in vivo experiments, sensitivity of TLR detection methods, whether astrocytes are exposed to microglia prior to analysis, and/or astrocyte purity. The latter is of particular concern given the notorious nature of microglia to hide beneath astrocyte monolayers in culture [68]. Approaches designed to obtain highly purified astrocytes (i.e. FACS or magnetic bead purification) may help to resolve these issues.
Astrocytes also express TLR9 and are responsive to CpG ODN [49, 61, 62, 69, 70]. CpG ODN treatment of astrocytes induced p38 MAPK activation and subsequent iNOS expression, which was found to be MyD88-dependent since these effects were not observed in primary astrocytes isolated from MyD88 KO mice [69]. Further investigation into the signal transduction pathways triggered in astrocytes following CpG ODN exposure revealed the activation of I-κB kinase (IKK) and c-Jun N-terminal kinase (JNK), the latter of which was shown to be pivotal for inducing cytokine and chemokine expression [70]. Astrocytes have also been reported to express TLR1, TLR5, TLR6, and TLR7/8, although less information is available regarding their functional roles [57, 61, 62, 69]. A recent study has demonstrated a functional role for TLR7 signaling in astrocyte activation and cross-talk with TLR9 pathways to regulate inflammatory mediator expression [57].
2.3 Oligodendrocytes
Compared to microglia and astrocytes, the TLR repertoire of oligodendrocytes is even more limited with cells reported to express TLR2 and TLR3 [38, 46, 71, 72] (Figure 2). The exact role of TLRs in oligodendrocytes remains unclear, but they may be involved in CNS repair by regulating inflammation, gliosis, and myelin sparing after injury [73, 74]. Indeed, TLR2 KO and TLR4-deficient mice displayed reduced myelination following spinal cord injury, suggesting that both receptors are necessary for proper remyelination. However, studies from the same group reveal differential effects of intraspinal microinjection of the TLR2 and TLR4 ligands zymosan and LPS, respectively, suggesting that TLR2 activation is not always neuroprotective and most likely depends on the CNS site and local microenvironment [74, 75].
2.4 Neurons
Recent studies have provided evidence that CNS neurons primarily express intracellular TLRs, namely TLR3, TLR7/8, and TLR9, suggesting a role for TLRs during both physiological and pathological conditions [14, 76–79]. Neurons have also been reported to express TLR4, which interacts with CD14 and either MD-1 or MD-2 at the cell surface [80, 81]; however, the functional impact of TLR4 signaling in neurons remains to be determined.
In addition to the inflammatory roles of TLRs, these receptors are now known to impact developmental regulation. For example, ex vivo treatment of embryonic cortical neurospheres with poly(I:C) reduced the number of proliferating cells and neural progenitor cell (NPC) formation; however, this effect was not observed in neurospheres prepared from TLR3 KO mice [82]. Likewise, TLR2 activation inhibited embryonic NPC proliferation, whereas TLR8 signaling attenuated neurite outgrowth and induced apoptosis [83, 84]. Collectively, these findings reveal a novel role for TLRs as regulators of NPC proliferation during development. However, the effects of TLR activation appear distinct between mature neurons versus NPCs. For example, TLR2 activation in vitro promotes neuron differentiation, while TLR4 signaling inhibits neuronal differentiation and self-renewal of NPCs [85]. Likewise, TLR2 KO mice exhibited impaired hippocampal neurogenesis in vivo, whereas TLR4-deficient mice demonstrated enhanced NPC proliferation and neuronal differentiation [85]. The impact of TLR signaling on CNS stem cell populations will be an intriguing area for future investigation based on the interest to manipulate these processes for therapeutic treatment of neurodegenerative disorders such as Alzheimer’s and Parkinson’s Disease.
3. Roles for TLRs During CNS Infection
3.1 Impact of TLRs during Bacterial Meningitis
Bacterial meningitis is an acute purulent infection of the leptomeninges and subarachnoid space. The introduction of antibiotics and childhood vaccinations has reduced the incidence and severity of bacterial meningitis; however, morbidity and mortality rates still remain high, particularly in underdeveloped countries [86]. The normal cerebrospinal fluid (CSF) milieu, which bathes the leptomeninges and subarachnoid space, is typically immunologically quiescent and inefficient at rapidly responding to bacterial infections. This is due to multiple factors, including a paucity of professional phagocytes for immediate bacterial recognition, low levels of opsonizing proteins and capsule-specific antibodies, in addition to the expression of immunosuppressive molecules that can impede pathogen uptake and/or killing [87].
Bacterial meningitis can be caused by numerous pathogens including Neisseria meningitidis, Streptococcus pneumoniae, Streptococcus agalactiae, Escherichia coli, Listeria monocytogenes, and Mycobacterium tuberculosis. Once pathogens have penetrated the subarachnoid space via the blood/CSF barrier, the inherent immune impaired nature of the CSF compartment allows for rapid bacterial replication. The host immune response is not activated until pathogen density becomes relatively high [88]. Numerous studies have established that the host immune response during meningitis, rather than the infectious agent itself, is largely responsible for the extensive tissue damage that occurs during infection [89, 90]. Therefore, therapies need to be directed towards both pathogen eradication with antibiotics as well as preventing the injurious effects of the ensuing immune response. The latter is currently achieved with steroid treatment; however, this is a rather non-specific approach and the targeting of specific harmful pathways cannot be realized.
Immune recognition of S. pneumoniae occurs via the coordinate action of multiple TLRs. TLR2 senses the bacterial cell wall components LTA and PGN as well as lipoproteins [39, 91, 92], whereas TLR4 mediates the immune response to pneumococci through its interaction with pneumolysin, a major virulence determinant of the organism [93, 94]. TLR9 has been implicated as another sensor for live S. pneumoniae via bacterial DNA recognition [95]. Further evidence of TLR cooperativity is revealed by the fact that a single deficiency of TLR2, TLR4, or TLR9 causes only selective and relatively modest reductions in cytokine production by pneumococci-stimulated immune cells [95, 96], whereas the combined loss of all three TLRs is essential for anti-bacterial immunity and recapitulates the phenotype of cells lacking the central TLR adaptor MyD88 [97, 98].
TLRs are also important for initiating host immunity in response to other bacterial meningitis strains. Namely, TLR2, TLR4, and TLR9 participate in N. meningitidis recognition [95]; however, Neisseria can also trigger immune cell activation in the absence of TLRs, suggesting that TLR-independent signaling pathways also contribute to Neisseria detection. With regard to other meningitis etiologic agents, TLR2 and possibly TLR7 and/or TLR8 have been implicated in sensing the group B streptococcus, S. agalactiae [99]. In addition, TLR2 and TLR5 have been shown to recognize LTA and flagellin from L. monocytogenes [100–102]. Collectively, these in vitro studies have demonstrated that distinct and often overlapping sets of TLRs, as well as other PRRs not discussed here, are used to sense major meningeal pathogens.
Some disagreement exists with regard to the functional impact of specific TLR pathways during bacterial meningitis, although collectively, the available data favor the action of multiple TLR pathways for eliciting maximal anti-bacterial immunity. For example, mice lacking TLR2 demonstrated increased bacterial burdens and TNF-α levels, which correlated with enhanced blood-brain barrier (BBB) disruption [103]. However, in a separate study, TLR2 only impacted early infection, whereas later stages of disease were not affected [104]. A more severe phenotype in MyD88 KO mice implicated other TLRs/IL-1R/IL-18R during meningitis [105]. This concept was reinforced in a subsequent study where TLR2 and TLR4 individually were found to play crucial roles in host defense and immune activation during Streptococcus pneumoniae meningitis [98]. However, the fact that immune responses in infected TLR2-TLR4 double KO mice were less impaired compared to MyD88 KO animals suggests that the more severe phenotype in the latter may be attributed, in part, to the loss of IL-1R and/or IL-18R signaling. Indeed, IL-1 and IL-18 are major cytokine products elicited by TLR signaling, and both cytokines have been shown to impact the course of bacterial meningitis [106, 107]. Therefore, TLR signaling by meningeal pathogens not only requires MyD88, but the adaptor is also essential for transducing signals emanating from the IL-1R and IL-18R, representing a positive feedback loop to augment inflammatory mediator production.
With regard to Gram-negative meningeal pathogens, both TLR4- and MyD88-dependent pathways are critical for microglial responses to Citrobacter koseri. Specifically, proinflammatory mediator release was significantly reduced in both TLR4 mutant and MyD88 KO primary microglia following C. koseri exposure [108]. In contrast, MyD88, but not TLR4, proved critical for CNS C. koseri infection in vivo. MyD88 KO mice were exquisitely sensitive to C. koseri, demonstrating enhanced mortality rates and significantly elevated bacterial burdens compared to wild type (WT) animals (Liu and Kielian, manuscript in revision). Interestingly, although early proinflammatory mediator release was MyD88-dependent, a role for MyD88-independent signaling was evident within 24 h, revealing a compensatory response to CNS C. koseri infection. In contrast, TLR4 did not significantly impact bacterial burdens or proinflammatory mediator production in response to C. koseri. Similar findings were obtained with primary astrocytes, where MyD88-dependent pathways were essential for chemokine release in response to intact C. koseri, whereas TLR4 was dispensable; implicating the involvement of alternative TLRs since highly enriched astrocytes did not produce detectable IL-1 upon bacterial exposure, which also signals via MyD88 (Liu and Kielian, manuscript in revision). Collectively, these findings demonstrate the importance of MyD88-dependent mechanisms in eliciting maximal proinflammatory responses, astrocyte activation, and bacterial containment during CNS C. koseri infection, as well as a late-phase MyD88-independent signaling pathway for cytokine/chemokine production. These findings indicate that numerous TLRs are responsible for eliciting maximal proinflammatory responses and bacterial containment during C. koseri infection. The identity of these receptors remains unknown but could involve the actions of TLR5, since Citrobacter is a flagellated species, or TLR9 via recognition of CpG DNA.
In summary, TLRs are critical triggers of the immune response during meningitis, with TLR signaling representing a key pathway for inflammatory mediator production. Due to dysregulated inflammatory cascades that have been implicated in the pathophysiology of bacterial meningitis, interference with TLR activity may represent a strategy for damping meningeal inflammation and improving disease outcome. However, murine meningitis models clearly demonstrated that disruptions in MyD88 or TLR2/4 were detrimental to the host; therefore, the risk of uncontrolled infection as a consequence of impaired bacterial eradication during TLR antagonist therapy may surpass any potential benefit. Thus, any potential antagonist therapies must be appropriately scrutinized.
3.2 Impact of TLRs in Bacterial Brain Abscess Formation
Brain abscesses differ from meningitis in several respects. The most obvious being the site of infection, with abscesses localized within the parenchyma in contrast to meningitis, which occurs in the subarachnoid space. Inherent to this dichotomy is the fact that the resident immune cells available to immediately respond to infection differ dramatically. Specifically, resident microglia and astrocytes are activated within hours following parenchymal infection, whereas immune responses elicited by resident macrophages in the meningeal space are delayed due to the immune impaired nature of the CSF. The fact that microglia express all of the TLRs identified to date implicates these cells as key sensors of bacterial invasion in the CNS parenchyma prior to peripheral immune cell recruitment [109, 110]. In addition, although astrocytes express a more limited array of TLRs, these cells are also capable of contributing to parenchymal immune responses and represent an important source of chemokines [60].
Brain abscesses are elicited by pyogenic bacteria and are typified by widespread edema and necrosis. Regardless of the inciting pathogen, the infected area becomes surrounded by a fibrotic capsule in an attempt to contain the infection and minimize additional bystander damage to surrounding brain parenchyma. Brain abscesses account for one in every 10,000 hospital admissions in the United States of an infectious disease nature, with the main etiologic agents being streptococcal strains and Staphylococcus aureus [111–114]. Brain abscesses most frequently develop from bacterial emboli originating from systemic sites of infection that become trapped within the cerebral vasculature [111]. However, additional modes of infection can also occur, including direct penetrating trauma, complication of neurosurgery [115, 116], or from proximal infections within the ear, sinuses, or teeth [117]. Compounding the severity of these infections is the recent emergence of antibiotic-resistant strains of bacteria, which are becoming more commonly associated with brain abscesses [112, 118–121].
To date, the number of studies investigating TLR pathways triggered by intact bacteria in microglia is limited, with the majority of reports utilizing purified PAMPs. We argue that it is important to query cellular responses to live pathogens as well as PAMPs since the former may elicit distinct profiles based on the impact of virulence factors produced by viable organisms. To this effect, previous work from our laboratory revealed that although TLR2 is pivotal for PGN recognition, inflammatory cytokine expression in response to intact S. aureus was qualitatively similar [122]. However, one exception was IL-12p40, which was exaggerated in TLR2 KO microglia following exposure to intact S. aureus [122]. We have recently found that the ability of intact bacteria to augment IL-12 family member expression in TLR2 KO microglia was specific for Gram-positive organisms, since numerous Gram-negative strains were unable to elicit exaggerated responses (Holley et al., manuscript in revision). Inhibition of phosphatidylinositol 3-kinase (PI3K), MAPK, and JNK signaling was capable of restoring exaggerated IL-12p40, p70, and IL-27 expression to levels observed in WT cells. Additionally, a TLR9 antagonist ablated the exaggerated IL-12 response in TLR2 KO microglia, suggesting that TLR9 drives cytokine production following exposure to intact bacteria that remains unchecked in the absence of TLR2 signaling (Holley et al., manuscript in revision). These findings have identified a novel pathway of TLR crosstalk and highlight the importance of examining cellular responses to intact bacteria in addition to purified PAMPs, since the former is more reflective of events encountered during CNS bacterial infection in vivo.
In contrast to TLR2, MyD88 expression is essential for microglial activation in response to both intact S. aureus and PGN, suggesting that other TLRs or autocrine/paracrine actions of cytokines that utilize MyD88, such as IL-1 and IL-18, are involved in eliciting maximal immune responses to additional PAMPs found in S. aureus [37, 123]. The dichotomy between TLR2 and MyD88 phenotypes in microglia following S. aureus exposure is highly reminiscent of the relevant importance of each molecule during brain abscess formation in vivo [37, 124] and speaks to the importance of microglia in sensing infection. Indeed, evidence to support a key role for CNS intrinsic cells in eliciting protective immunity to S. aureus infection was recently demonstrated by our laboratory using MyD88 bone marrow chimeras [109].
Recent studies using a mouse experimental brain abscess model have revealed complex roles for TLRs in disease pathogenesis [125–127]. Interestingly, TLR2 had limited impact on the innate immune response during the acute stage of brain abscess formation induced by S. aureus, but influenced several aspects of adaptive immunity. This was reflected by elevated IL-17 expression in TLR2 KO mice, which correlated with a significant increase in IL-17-producing T cells [37, 122, 125]. Further analysis of abscess-associated T cell populations in TLR2 KO mice revealed an increase in both natural killer T (NKT) and γδ T cell infiltrates following CNS S. aureus infection [126], and both populations produced more IL-17 and IFN-γ compared to WT cells. It has been proposed that this heightened IL-17 response in TLR2 KO mice functions as a compensatory mechanism to control brain abscess pathogenesis, which appears plausible since IL-17 is a potent inducer of neutrophil chemokines and neutrophils are essential for bacterial containment in brain abscesses [128–130]. Macrophages represent one potential target of IL-17 action, since the cytokine was found to enhance TNF-α-induced CXCL2 and CCL2 expression, whereas glia were rather non-responsive to IL-17 [125].
Separate studies by Stenzel et al. have also shown a role for TLR2 and TLR4 in S. aureus-induced brain abscesses. Specifically, TLR2 and TLR4 KO mice demonstrated increased mortality rates concomitant with impaired bacterial clearance and enhanced immune cell recruitment into the infected CNS [127]. Although our initial report with TLR2 KO mice reported no differences in bacterial burdens, a subsequent study confirmed the findings from Stenzel et al., where S. aureus titers were elevated in brain abscesses of TLR2 KO animals [126], which likely stemmed from the use of different S. aureus strains. Namely, our earlier report utilized a lab adapted strain, whereas recent studies have utilized a S. aureus isolate that was recovered from a patient who died of a brain abscess [131]. Similarly, a clinical S. aureus isolate was utilized by Stenzel et al., providing further support of this possibility. It is more difficult to envision the mechanism of TLR4 action during S. aureus-induced brain abscess development since this is a Gram-positive organism that lacks the TLR4 ligand LPS. However, one possibility is that TLR4 recognizes endogenous “danger” signals released during necrosis. Indeed, numerous endogenous ligands have been identified for TLR4, including fibronectin fragments and heat shock proteins [2].
Unlike the more subtle phenotypes observed with TLR2, the TIR adaptor MyD88 is essential for generating a protective immune response during brain abscess development. Specifically, MyD88 KO mice were exquisitely sensitive to infection, succumbing within 48 h following bacterial exposure [124]. This was associated with global impairments in proinflammatory cytokine and chemokine production, resulting in a failure to recruit significant numbers of neutrophils and macrophages into the parenchyma [124]. This severe phenotype is likely due to the simultaneous loss of several key MyD88-dependent receptors, including multiple TLRs as well as the IL-1R and/or IL-18R. Evidence to support a role for IL-1R signaling is provided by the finding that IL-1 KO animals also demonstrated increased mortality and bacterial burdens compared to WT animals [132]. In addition, recent studies from our laboratory have established that IL-1R KO mice are also highly sensitive to CNS S. aureus infection, with animals succumbing to infection within 18–24 h, concomitant with significant decreases in neutrophil and macrophage influx into the brain and elevated bacterial burdens (Xiong and Kielian, unpublished observations). Of note, we have not found an important role for TLR9 signaling during brain abscess development (Kielian, unpublished observations). Collectively, these results suggest that TLR2 and IL-1R signaling are pivotal in regulating the anti-bacterial inflammatory response during brain abscess development. It is envisioned that understanding the roles for TLRs in both resident CNS glia as well as infiltrating immune cells will provide insights into how the immune response to bacterial infection can be tailored to achieve effective pathogen destruction without inducing excessive bystander damage of surrounding brain parenchyma. The relative impact of individual TLRs in CNS intrinsic versus extrinsic cells can be achieved with the use of radiation bone marrow chimera mice, an approach we have recently used to establish the critical importance for MyD88-dependent signaling in CNS resident cells for the generation of a WT immune response during brain abscess development [109].
3.3 Impact of TLRs during Viral Infection
Four TLRs, namely TLR3, TLR7, TLR8, and TLR9, recognize viral nucleic acids and are localized primarily in endosomal compartments [2]. As mentioned earlier, TLR3 senses dsRNA, TLR7/8 recognizes ssRNA, and TLR9 engages nonmethylated CpG motifs characteristic of microbial DNA. To date, the majority of work investigating the importance of TLRs in viral infections affecting the CNS has focused on TLR3 activation. In addition to sensing dsRNA viruses, TLR3 may also detect dsRNA intermediates that form during the replication cycle of ssRNA and DNA viruses [133, 134]. TLR3 and TLR7 can also recognize host RNA released following tissue damage, which has been implicated in autoimmunity [135, 136]. TLR3 utilizes the adaptor protein TRIF to elicit type I IFN production necessary for anti-viral responses, whereas TLR7 signals via MyD88 [133, 137].
Herpes simplex virus-1 (HSV-1) infections are widespread, and seropositivity may exceed 70% of the world population [138]. The virus is transmitted primarily by contact between skin or mucosa with contaminated oral secretions. Primary infections are usually acquired during childhood and often present as mild, self-limiting pharyngitis or are asymptomatic. However, in rare cases HSV-1 can spontaneously spread to the brain, causing herpetic encephalitis, a dangerous infection that can lead to death [139–142]. After HSV-1 replicates in the skin and mucosa, it reaches dorsal root ganglia termini, whereupon the virus is intraxonally transported to the trigeminal ganglia (TG), where it becomes latent. HSV-1 can be reactivated by numerous stimuli, including psychological stress, UV irradiation, and/or trauma [143–145]. These infections can be very severe and painful and are often a cause of morbidity in immunocompromised individuals with cancer, AIDS, severe burns, and patients receiving immunosuppressive therapies [146].
Recent studies have shown that HSV infection signals via TLR2, TLR4, and TLR9 to elicit chemokine synthesis and regulate immune responses in the TG and brain [147, 148]. Specifically, TLR2 and TLR4 KO mice were unable to produce monocyte chemoattractant protein-1 (MCP-1/CCL2) following HSV-1 exposure. This coincided with a reduction in immune cell infiltrates at the site of infection and increased viral load [147, 149]. Indirect evidence for TLR2 and TLR9 in a rat model of HSV encephalitis was demonstrated by the finding that rat strains which are permissive for viral infection did not display elevations in TLR2 and TLR9 expression, whereas strains resistant to viral infection did [150]. This heightened expression of TLR2 and TLR9 is most likely explained by increased phagocyte recruitment into the CNS, as HSV susceptible rat strains showed delayed phagocytic cell infiltration into sites of infection. A separate study demonstrated that TLR2 and TLR9 act in a synergistic manner to influence innate anti-viral responses and protect against HSV-1 infection in the brain [151]. Therefore, it appears as if TLRs modulate HSV-1 invasion, as TLR deficiencies lead to defects in inflammation and phagocyte recruitment, which consequently allows virus entry into the CNS from peripheral tissues [152].
West Nile virus (WNV) is another CNS tropic virus that requires TLR3 for BBB penetration and subsequent CNS invasion [153]. WNV has a predilection for CNS colonization by its ability to traverse endothelial cell tight junctions and astrocyte end feet at the BBB. The mechanism of TLR3 action occurs in the periphery, whereby TLR3 signaling augmented systemic TNF-α levels, which led to a transient increase in BBB permeability and permitted virus invasion into the CNS [153]. However, a recent report demonstrated that TLR3 offered protection against WNV encephalitis [154]. The reasons for these discrepancies are not clear, but may result from differences in viral propagation methods or infectious inoculums utilized. MyD88 KO mice were highly susceptible to WNV infection with impaired immune cell recruitment, suggesting the coordinate action of multiple TLRs and/or IL-1R/IL-18R [155]. A recent study has implicated the Toll/interleukin-1 receptor (TIR)-containing adaptor protein sterile alpha and HEAT/Armadillo motif (SARM) in CNS WNV infection. SARM is preferentially expressed in CNS resident cells and a report utilizing SARM KO mice has demonstrated that WNV replication is markedly increased only in the brainstem of these animals, which was associated with enhanced mortality [156]. SARM deficiency was also linked to reduced TNF-α expression and it appears that SARM functions to restrict viral infection and neuronal injury in a brain region-specific manner, most likely by modulating the activation of resident CNS inflammatory cells [156].
3.4 Impact of TLRs during CNS Parasitic Infections
Parasite infections affecting the CNS are a major cause of morbidity and mortality worldwide and discovering novel therapies to control these infections requires an understanding of tissue-specific host responses [157]. A growing body of literature indicates that TLRs modulate host inflammatory responses to CNS parasitic infections such as sleeping sickness, toxoplasmosis, river blindness, and neurocysticercosis [77, 158–160]. Interestingly, TLR activity can exert differential effects depending on the context of infection, which further complicates interpretation of their role. For example, TLR activation can be important for parasite clearance and host survival; however, TLR-mediated responses are also capable of exacerbating disease severity and subsequent tissue damage [157].
In a murine model of neurocysticercosis, the expression of nearly all TLRs was upregulated in the brain during infection [77]. TLR expression was differentially distributed among various CNS intrinsic and infiltrating cell types, with TLR2 localized to resident CNS cells, particularly astrocytes, whereas TLR1 and TLR9 were primarily expressed in infiltrating leukocytes. These findings suggested the potential involvement of numerous TLRs in modulating CNS immune responses to the parasite. Surprisingly, MyD88 KO mice displayed decreased disease severity and less mortality compared to WT animals in the neurocysticercosis model [161]. CNS tissues of MyD88 KO mice revealed less parenchymal damage and lacked prominent microglial and astrocyte activation that is typically seen during infection of WT animals. Likewise, infected MyD88 KO animals exhibited reduced numbers of infiltrating immune cells and less TNF-α, IFN-γ and IL-6 production compared to WT mice. The latter could explain why BBB integrity was less severe in MyD88 KO mice following parasite exposure. Together, these findings suggest that MyD88-mediated signals play a critical role in the hyper-inflammatory response that contributes to neuropathology and disease severity during neurocysticercosis [161]. Whether this is mediated by the individual or combined actions of TLRs, IL-1R, or IL-18R remains to be determined.
In a murine model of cerebral malaria (CM), CNS pathology was shown to be MyD88-dependent but was not affected by single deficiencies in TLR1, −2, −3, −4, −6, −7, or −9 signaling [162, 163]. Similar findings were obtained with mice deficient in the adapter proteins MyD88, TIRAP, and TRIF as well as triple TLR2/4/9-deficient mice, suggesting that innate immune activation and CM development involves the coordinate action of multiple TLR signaling pathways. In contrast, other reports have demonstrated that MyD88-dependent signaling is deleterious during CM, as evidenced by increased survival of MyD88 but not TRIF KO animals, the latter of which signals via a MyD88-independent pathway [164]. Another study has suggested that development of lethal CM is influenced by the genetic background of MyD88 KO mice [165]. Specifically, MyD88 deletion on the susceptible C57BL/6 background resulted in resistance to CM, whereas removal of MyD88 on the resistant BALB/c background led to increased mortality. The mechanism(s) responsible for this strain-dependent outcome appear to involve effects of TLR signaling on cytokine production and Treg numbers [165].
In summary, TLR activation during the course of CNS parasitic infections can be seen as a “double edged sword”, capable of benefiting the host as well as contributing to CNS pathology. This highlights the dual role for TLRs during CNS infections, first to facilitate the initial response to the invading pathogen and second, by recognizing newly liberated self-molecules resulting from tissue injury. Some of these endogenous molecules may further trigger TLR activation and exacerbate inflammation, resulting in bystander destruction of surrounding parenchyma.
4. Evidence for TLRs in Neuronal Injury
Ischemic brain injury typically arises following arterial embolism, culminating in the loss of blood flow to a specific brain region [166]. The resultant metabolic stress from nutrient deprivation induces widespread cell death [167]. In addition, tissue surrounding the ischemic core, referred to as the penumbra, is also affected by inflammation [168]. The regulation of inflammation after stroke is complex, involving numerous cellular processes in different cell types. Infarcted brain tissue is typified by neutrophil and macrophage infiltrates and glial activation at the site of injury as well as in the surrounding penumbra. Several groups have demonstrated a role for TLRs in the brain following neuronal injury, which suggests a more diverse role beyond pathogen recognition.
TLR expression increases during inflammatory conditions associated with the cerebral vasculature, such as aneurysm [169]. For example, TLR4 expression on cerebral vascular endothelium increases following sub-arachnoid hemorrhage and TLR4 deficiency has been shown to be important in the recruitment of neutrophils expressing myeloperoxidase (MPO) following transient focal cerebral ischemia [170, 171]. Both TLR2 and TLR4 have been implicated in mediating neuronal death during stroke as infarct size was significantly reduced in TLR2 and TLR4 KO mice [172–174]. In contrast, TLR3 and TLR9 do not appear to impact stroke pathogenesis [174]. Microglia have been implicated as mediators of neuronal death following stroke, since microglial TLR2 expression is enhanced during experimental ischemia [76]. However, whether increased microglial TLR2 expression was simply a general marker of inflammation or functionally impacted disease remained to be determined. Evidence to support the latter was provided by the fact that infarct size, neurological deficits, and neuronal damage were significantly attenuated in TLR2 and TLR4 KO mice compared to WT animals following ischemia/reperfusion injury [76]. However, a recent report has demonstrated that disruption of MyD88 or TRIF signaling did not impact infarct size in a mouse model of middle cerebral artery occlusion or neuronal viability following oxygen-glucose deprivation in vitro, suggesting that alternative adaptors are responsible for mediating TLR signaling in cerebral ischemia [175]. Collectively, these studies implicate TLRs in exerting detrimental effects following stroke. The identity of the endogenous molecular triggers responsible for TLR activation in the context of stroke have yet to be defined, but are likely numerous based on the extensive inflammation and neuronal injury that ensues following the ischemic event.
5. Alzheimer’s Disease
Several changes in the brain have been associated with aging, including a progressive increase in the basal neuroinflammatory state, elevations in innate immune receptor expression, as well as the appearance of dystrophic microglia with fragmented processes [176–178]. The reasons for these changes are currently unknown but conceivably could represent an adaptive response to aging, which progresses over time. Indeed, microglial phagocytosis of amyloid beta (Aβ) has been suggested to positively impact Alzheimer’s disease (AD) pathogenesis by facilitating Aβ clearance from the brain [179, 180]. Proinflammatory cytokine levels are related to the magnitude of plaque burden in the AD brain, while it has also been suggested that the inflammatory response facilitates Aβ production and deposition [181].
Microglia surrounding Aβ deposits display an activated phenotype and extended processes which envelop the plaque [182, 183]. Microglia interact with fibrillar β-amyloid (fAβ) through an ensemble of surface receptors composed of the alpha(6)beta(1) integrin CD36, CD47, and the class A scavenger receptor [184]. Evidence indicates that TLRs also function as members of the microglial fAβ receptor complex [185–189] and a recent study demonstrated that Aβ triggers microglial inflammatory mediator production via TLR4-TLR6 heterodimers whose assembly is regulated by CD36 [190]. Treatment of microglia with AD plaque material induced strong upregulation of TLR2, 4, 5, 7, and 9 mRNA compared to age-matched plaque-free tissue [188]. In addition, TLR4-deficient mice displayed increases in diffuse and fAβ deposits compared to WT mice [187], which correlated with elevated TNF-α, IL-1β, IL-10, IL-17, CD11b and GFAP expression, suggesting that TLR4 signaling is involved in AD progression and could be a new therapeutic target for AD [191]. In addition, TLR2 loss exacerbated memory deficits in a mouse AD model, which could be countered by reconstitution of bone marrow from TLR2 WT mice, suggesting that infiltrating leukocytes provide beneficial functions, likely via phagocytic clearance of Aβ [192]. In contrast, in vitro stimulation of microglia lacking CD14, TLR4 or TLR2 could not initiate the signaling cascades required for reactive oxygen species production and phagocytosis in response to fAβ [185–187]. Another study has determined that TLR4 signaling increases the vulnerability of neurons to Aβ and oxidative stress [193]. Administration of the TLR9 agonist CpG DNA reduced cortical and vascular Aβ levels and negated learning deficits in a mouse APP Tg model [194]. Finally, a recent study has demonstrated that cognitive impairment was less severe in TLR2/4 double KO mice following Aβ active immunization, which correlated with reductions in proinflammatory mediator expression [195]. Therefore, similar to many of the other diseases already discussed, the summation of current evidence suggests that TLRs may either positively or negatively impact cellular responses during AD. The outcome of TLR signaling may depend on numerous factors including plaque burden, the biochemical nature of Aβ, and extent of neuronal pathology; however, all of these possibilities remain speculative at the present time.
6. Multiple Sclerosis
Multiple sclerosis (MS) is a chronic and debilitating disease characterized by autoimmune demyelination and progressive axonal degeneration within the CNS. The most widely utilized animal model for MS, experimental autoimmune encephalitis (EAE), has demonstrated a role for PAMPs in inducing clinical symptoms of disease. In particular, early studies established that both mycobacterial components and pertussis toxin were required for disease development following immunization with myelin antigens [196]. Other studies demonstrated that EAE could be induced without pertussis toxin using multiple injections of myelin components in complete Freund’s adjuvant (CFA), which also contains mycobacterial components thought to trigger TLR signaling [197]. Indeed, mice immunized with myelin peptides in the presence of incomplete Freund’s adjuvant (IFA) do not develop EAE, but do develop disease when Mycobacterium tuberculosis is added to the adjuvant [198]. More recently, it has become clear that other adjuvants such as CpG DNA, a ligand for TLR9, can also stimulate EAE induction [198–200].
Several studies support a role for TLRs in modulating EAE. First, the TLR2 ligand PGN was shown to stimulate EAE development in conjunction with IFA [201]. In addition, TLR2 KO mice were susceptible to myelin oligodendrocyte glycoprotein (MOG)-induced EAE, whereas mice deficient for MyD88 were resistant to disease development [202]. The resistance of MyD88 KO mice to EAE was determined to be dependent, in part, on TLR9 signaling, since EAE onset was delayed in animals where TLR9 was specifically deleted within the CNS compartment. In addition to resistance to active MOG-induced EAE, MyD88 KO mice were also refractory to EAE following the adoptive transfer of encephalitogenic MOG-specific T cells, the latter of which bypasses TLR adjuvant signaling [203]. The resistance of MyD88 KO mice during adoptive transfer EAE depended on IL-10 induction, since susceptibility to adoptive transfer EAE was restored in MyD88/IL-10 double KO mice. Similarly, adoptive transfer of IL-10 secreting MOG-specific T cells from MyD88 KO mice was capable of suppressing disease in WT animals, indicating a direct role for MyD88 signaling in dictating T cell cytokine phenotype [203]. Based on the available evidence, MyD88 signaling may represent a possible target for future MS therapies. However, it should be noted that TLR9 and TLR4 KO mice were recently shown to exhibit more severe EAE, which suggests the existence of regulatory roles for both receptors in regulating encephalogenic responses [204]. Additional studies are needed to define the conditions where TLR signaling may either positively or negatively impact the course of EAE and mechanisms involved.
7. Spinal Cord Injury
In the United States, the incidence of spinal cord injury (SCI) has been estimated at 12,000 new cases per year [205]. The leading causes of SCIs are motor vehicle accidents, falls, sporting/recreation accidents, and violence [206, 207]. Traumatic SCI commonly results from fractured or dislocated vertebrae, which contorts/compresses the spinal cord. Non-traumatic spinal compression can also occur during surgical intervention, tumor invasion, or degenerative bone disease [208–210]. During trauma, axon and myelin damage is delayed 24 to 48 h post-injury; moreover, it has been suggested that this delayed neurodegeneration post-injury could be attenuated by blocking secondary injury cascades, such as ischemia, excitotoxicity and inflammation [211, 212]. Recruitment of inflammatory leukocytes to the injured spinal cord is a normal physiological response associated with the production of cytokines and proteinases that are involved in host defense and wound repair. Although required for debris scavenging and preparation of the injury site for the proliferative and remodeling phases of wound repair, the physiological functions of neutrophils, macrophages and lymphocytes may, in some cases, also contribute to CNS tissue damage.
Microinjection of the TLR2 agonist zymosan into the spinal cord elicits the production of neurotoxic mediators from CNS macrophages [213, 214]. Although TLR4 signaling also induces macrophage activation within the spinal cord, only TLR2 causes significant axonal and myelin damage [75]. Interestingly, recent reports have suggested that the nature of the TLR stimulus may dictate neuroprotective versus neurodestructive outcomes. For example, data indicate that TLR engagement by, as of yet unidentified DAMPs, is critical for neuroprotection, axon regeneration, and cell replacement following spinal cord injury [14, 74, 75, 84, 85, 215, 216]. Furthermore, deficiencies in TLR2 or TLR4 signaling exacerbated post-traumatic accumulation of CNS macrophages that was accompanied by functional impairment and excessive tissue pathology [74]. These studies suggest that TLR activation represents a neuroprotective mechanism in the context of SCI; however, the mechanisms of action remain to be elucidated. Currently, we are only beginning to decipher the impact of TLRs and other receptors on SCI pathology and repair, yet the existing literature is clear that TLRs influence post-traumatic inflammation, neuron survival, and axon regeneration and may represent promising targets for modulating SCI to facilitate reparative processes. If we can discern how TLRs control neural and glial progenitor cell fate, it may be possible to utilize these receptors in cell replacement therapy for SCI and other neurological disorders [217, 218].
8. TLRs in Neurogenesis, Learning and Memory
In addition to their well appreciated roles in initiating inflammatory responses to microbial stimuli, recent evidence has implicated TLRs in CNS cellular repair in adults and development during fetal life in the absence of pathogens. A recent review has also highlighted the possibility that TLR signaling within the CNS may represent a secondary adaptation to attenuate inappropriate immune responses following acute injury or chronic disease. In this case, TLR action would quell deleterious responses that would interfere with repair processes to promote tissue homeostasis [219]. Here we summarize the available data on the expression and function of individual TLR family members in the healthy CNS and why TLRs deserve close scrutiny as a novel group of therapeutic targets for CNS disorders. For a more in depth discussion regarding TLRs and neuroplasticity, readers are directed to a recent review that covers this literature more extensively [220].
8.1 Learning and Memory
Recent studies have implicated TLR4 in the pathogenesis of cognitive impairment that often accompanies systemic inflammation/bacterial sepsis [221] as well as in AD [76, 187]. Similarly, TLR2 and TLR4 signaling have also been associated with impaired functional outcome after focal ischemia [76]. A few reports have investigated the connection of TLRs and behavioral/cognitive function, with TLR3 affecting both hippocampal-dependent and independent behaviors [222]. For example, adult TLR3 KO mice exhibit enhanced hippocampal-dependent working memory in Morris water maze, novel object recognition, and contextual fear-conditioning tasks while demonstrating impaired amygdale-related behavior and anxiety in the cued fear-conditioning, open field, and elevated plus maze tasks [222]. However, intracerebroventricular infusion of a TLR3 ligand impaired working memory, but not reference memory [222]. The mechanisms responsible for the impact of TLR3 on learning and memory remain to be identified; nonetheless, these studies highlight the fact that TLR signaling impacts normal homeostatic responses. It appears plausible that TLR2, TLR4, and TLR8 may influence behavior/cognitive function since they have been shown to impact NPC proliferation (TLR2 and TLR4) and neuron survival (TLR8). However, additional studies are needed to address this point.
8.2 Neurogenesis
Neural progenitor cells (NPCs) can express immune-relevant molecules, such as cell adhesion molecules, chemokine receptors, and inflammatory cytokines that enable them to functionally interact with an inflamed CNS microenvironment [223–227]. TLR2 is expressed on cells in areas of the adult brain associated with the generation of new neurons, namely the subventricular zone (SVZ) of the lateral ventricles and the subgranular zone (SGZ) of the hippocampal dentate gyrus [83, 85]. Further examination revealed that both TLR2 and TLR4 are expressed on adult NPCs [228] and have opposing effects on NPC proliferation and differentiation. Specifically, TLR2 KO mice demonstrated impaired hippocampal neurogenesis, whereas TLR4 KO animals displayed enhanced proliferation and neuronal differentiation. Similar to TLR4 KO mice, TLR3 KO animals exhibited increased hippocampal neurogenesis, in addition to increased hippocampal CA1 and dentate gyrus volumes [222]. Likewise, NPCs from TLR3 KO embryos formed greater numbers of neurospheres compared to WT embryos and the number of proliferating cells were also increased in the cortex of TLR3 KO mice [82]. Since these observations were made in the absence of any known infectious stimuli, it remains to be determined what signals are responsible for these phenotypes following TLR loss. Regardless, these results do highlight the complexity of TLR actions and implies that manipulation of adult stem cells could theoretically be achieved by modulating TLR activity, although this remains highly speculative. Finally, TLR2 or TLR4 activation of NSCs induces proinflammatory cytokine secretion and suggests that NSCs may be primed to participate in cytokine production during neuroinflammatory or traumatic conditions [228].
9. Conclusions and Outstanding Questions
The current evidence to date suggests that TLR-mediated responses can exert either beneficial or detrimental effects, depending on the strength and timing of the activating signal. Yet, the impact of TLRs in the CNS extends well beyond their role in controlling host defense responses. These receptors play important roles in tissue development, cellular migration and differentiation, limiting inflammation, and mounting repair processes following trauma (Table 2). It appears likely that endogenous ligands are particularly relevant to activate these TLR functions during development or when microbial invaders are not present. Although the ligand(s) responsible for triggering TLR involvement in the absence of infectious insults have not yet been elucidated, it is apparent that these PRRs play a role, at some level, in influencing responses to injury/trauma and subsequent regenerative responses. One point of interest is the apparent “double edged sword” potential that TLR signaling can exert across several CNS disorders, including infectious as well as non-infectious diseases. This suggests that TLR activation may be subject to rheostat control, where chronic triggers would push the system towards tissue pathology, whereas limited responses would return the system to a homeostatic balance. Of note, although interrogating the functional role of TLRs using KO mice is useful, it should be acknowledged that the absence of particular TLRs during development may impact later responses in the adult and/or elicit compensatory reactions that are normally not operative. In this case, it would be useful to examine cell type-specific and/or inducible KO systems to better fine tune the system and allow a kinetic assessment of receptor activity to be assessed.
Table 2.
Toll-like receptors in health and disease in the CNS
| Healthy CNS | TLR(s) | Mechanism | References |
|---|---|---|---|
| Memory | 3 | TLR3 KO mice exhibit enhanced hippocainpal-dependent working memory | 34, 203 |
| Neurogenesis | 2, 3, & 4 | TLR KO mice exhibit impaired (TLR2) or enhanced (TLR3 & 4) neurogenesis | 32, 118, 157 |
| CNS. Insult | TLR(s) | Pathology | |
| Bacteria Meningitis | 2,4 & 9 | TLRs are necessary for eliciting maximal anti-bacterial immunity | 45, 146 |
| Bacterial Abscess | 2 & 4 | TLRs are necessary for eliciting maximal anli-bacterial immunity | 96, 151, 192 |
| Viral Infection | 3 & 9 | LLRs function to resteic viral infection and neuronal injury | 87, 220 |
| Parasitic Infection | 1,2 & 9 | TLRs contribute to parasite clearance: but may also exacerbate disease severity | 29, 62, 145 |
| Neuronal Injury | 2 & 4 | TLR2 and TLR4 have been implicated in mediating neuronal death during stroke | 122, 124, 233 |
| Spinal Cord Injun’ | 2 & 4 | TI.R2 stimulation causes significant axonal and myelin damage | 98, 183 |
| Alzheimer’s | 2, 4, 5, 7, & 9 | TLE deficient mice demonstrate increases in diffuse and fibrillar Aβ deposits | 55, 170, 197, 209 |
It is intriguing that similar TLR combinations have been implicated in recognizing diverse infectious agents, which raises the question of how immune responses are tailored for optimal eradication of specific pathogens. One possibility that could facilitate specificity is the paired action of TLRs with other PRRs that together, dictate a custom secretory response for the particular pathogen in question. Although a few examples exist with regard to TLR cooperativity with alternative PRRs [229, 230], this issue is relatively underexplored in the context of CNS infection and warrants further investigation.
A related issue that remains unresolved is how TLR responses are tailored to discriminate between PAMPs and DAMPs and ensure the correct intensity and duration of cytokine/chemokine signaling that is appropriate for the inciting stimulus. Similarly, although mechanisms leading to termination of TLR signaling are beginning to be identified in immune cell populations, not much information is available in this regard in CNS cell types. Examples include molecules that directly interact with intermediates of the TLR signaling pathway to halt the cascade, subcellular compartmentalization and ubiquitination of TLRs to control their activity, and expression of other regulatory molecules such as the suppressors of cytokine signaling (SOCS) proteins [231–233]. Although much attention has focused on ways to trigger TLR activation within the CNS compartment, it could be argued that a better understanding as to how signals via these receptors are terminated may uncover novel means to manipulate TLR activity during CNS disease.
Another issue that has received scant attention is the impact of TLR action at the BBB. Although some work has been done to demonstrate that systemic TLR3 signaling is required for WNV penetration of the BBB, few studies have examined TLR effects on cells that constitute the BBB. Several TLRs are expressed on rat and human cerebral endothelial cells, including TLR2, TLR3, TLR4, and TLR6, which are significantly enhanced following exposure to oxidative stress or TNF-α [234, 235]. However, the impact of TLR engagement at the BBB remains to be determined but is expected to be an attractive therapeutic target given the basic requirement for pathogen and immune cell penetration across the BBB during a wide array of CNS diseases.
Manipulation of TLR signaling has immense therapeutic possibility, but at the same time is also fraught with considerable risk. For example, the ability of TLR ligands to trigger adaptive immune responses may identify novel adjuvants and potential for vaccine design and manipulation, yet conversely may also exacerbate underlying inflammatory disorders such as atherosclerosis and autoimmune diseases. Additional studies addressing these questions within the CNS microenvironment are warranted.
Acknowledgements
This work was supported by the NIH National Institute of Neurological Disorders and Stroke (NINDS) R01 NS040730, R01 NS055385, and R01 NS053487 to T.K. Due to space constraints it was not possible to cite all original studies on this topic. The authors thank Kari Nelson for editorial assistance.
References
- 1.Medzhitov R, Janeway C. Innate immune recognition: mechanisms and pathways. Immunol. Rev. 2000;173:89–97. doi: 10.1034/j.1600-065x.2000.917309.x. [DOI] [PubMed] [Google Scholar]
- 2.Takeuchi O, Akira S. Pattern recognition receptors and inflammation. Cell. 2010;140:805–820. doi: 10.1016/j.cell.2010.01.022. [DOI] [PubMed] [Google Scholar]
- 3.Kaisho T, Akira S. Pleiotropic function of Toll-like receptors. Microbes Infect. 2004;6:1388–1394. doi: 10.1016/j.micinf.2004.08.019. [DOI] [PubMed] [Google Scholar]
- 4.Qureshi ST, Medzhitov R. Toll-like receptors and their role in experimental models of microbial infection. Genes Immun. 2003;4:87–94. doi: 10.1038/sj.gene.6363937. [DOI] [PubMed] [Google Scholar]
- 5.Hoebe K, Beutler B. LPS, dsRNA and the interferon bridge to adaptive immune responses: Trif, Tram, and other TIR adaptor proteins. J. Endotoxin Res. 2004;10:130–136. doi: 10.1179/096805104225004031. [DOI] [PubMed] [Google Scholar]
- 6.Pasare C, Medzhitov R. Toll-like receptors: balancing host resistance with immune tolerance. Curr. Opin. Immunol. 2003;15:677–682. doi: 10.1016/j.coi.2003.09.002. [DOI] [PubMed] [Google Scholar]
- 7.Hertz CJ, Kiertscher SM, Godowski PJ, Bouis DA, Norgard MV, Roth MD, Modlin RL. Microbial lipopeptides stimulate dendritic cell maturation via Toll-like receptor 2. J. Immunol. 2001;166:2444–2450. doi: 10.4049/jimmunol.166.4.2444. [DOI] [PubMed] [Google Scholar]
- 8.Boonstra A, Asselin-Paturel C, Gilliet M, Crain C, Trinchieri G, Liu YJ, O'Garra A. Flexibility of mouse classical and plasmacytoid-derived dendritic cells in directing T helper type 1 and 2 cell development: dependency on antigen dose and differential toll-like receptor ligation. J. Exp. Med. 2003;197:101–109. doi: 10.1084/jem.20021908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kirschning CJ, Schumann RR. TLR2: cellular sensor for microbial and endogenous molecular patterns. Curr. Top. Microbiol. Immunol. 2002;270 doi: 10.1007/978-3-642-59430-4_8. [DOI] [PubMed] [Google Scholar]
- 10.Piccinini AM, Midwood KS. DAMPening inflammation by modulating TLR signalling. Mediators Inflamm. 2010 doi: 10.1155/2010/672395. PMID: 20706656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Karikó K, Ni H, Capodici J, Lamphier M, Weissman D. mRNA is an endogenous ligand for Toll-like receptor 3. J. Biol. Chem. 2004;279:12542–12550. doi: 10.1074/jbc.M310175200. [DOI] [PubMed] [Google Scholar]
- 12.Kielian T. Toll-like receptors in central nervous system glial inflammation and homeostasis. J. Neurosci. Res. 2006;83:711–730. doi: 10.1002/jnr.20767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lafon M, Megret F, Lafage M, Prehaud C. The innate immune facet of brain: Human neurons express TLR3 and sense viral dsRNA. J. Mol. Neurosci. 2006;29:185–194. doi: 10.1385/JMN:29:3:185. [DOI] [PubMed] [Google Scholar]
- 14.Cameron JS, Alexopoulou L, Sloane JA, DiBernardo AB, Ma Y, Kosaras B, Flavell R, Strittmatter SM, Volpe J, Sidman R, Vartanian T. Toll-Like Receptor 3 Is a Potent Negative Regulator of Axonal Growth in Mammals. J. Neurosci. 2007;27:13033–13041. doi: 10.1523/JNEUROSCI.4290-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Farina C, Krumbholz M, Giese T, Hartmann G, Aloisi F, Meinl E. Preferential expression and function of Toll-like receptor 3 in human astrocytes. J. Neuroimmunol. 2005;159:12–19. doi: 10.1016/j.jneuroim.2004.09.009. [DOI] [PubMed] [Google Scholar]
- 16.Lauw FN, Caffrey DR, Golenbock DT. Of mice and man: TLR11 (finally) finds profilin. Trends Immunol. 2005;26:509–511. doi: 10.1016/j.it.2005.08.006. [DOI] [PubMed] [Google Scholar]
- 17.Yarovinsky F, Zhang D, Andersen JF, Bannenberg GL, Serhan CN, Hayden MS, Hieny S, Sutterwala FS, Flavell RA, Ghosh S, Sher A. TLR11 activation of dendritic cells by a protozoan profilin-like protein. Science. 2005;308:1626–1629. doi: 10.1126/science.1109893. [DOI] [PubMed] [Google Scholar]
- 18.Zhang D, Zhang G, Hayden MS, Greenblatt MB, Bussey C, Flavell RA, Ghosh S. A toll-like receptor that prevents infection by uropathogenic bacteria. Science. 2004;303:1522–1526. doi: 10.1126/science.1094351. [DOI] [PubMed] [Google Scholar]
- 19.Alexopoulou L, Holt AC, Medzhitov R, Flavell RA. Recognition of double-stranded RNA and activation of NF-kappaB by Toll-like receptor 3. Nature. 2001;413:732–738. doi: 10.1038/35099560. [DOI] [PubMed] [Google Scholar]
- 20.Takeda K, Kaisho T, Akira S. Toll-like receptors. Annu. Rev. Immunol. 2003;21:335–376. doi: 10.1146/annurev.immunol.21.120601.141126. [DOI] [PubMed] [Google Scholar]
- 21.Coll RC, O'Neill LA. New insights into the regulation of signalling by toll-like receptors and nod-like receptors. J. Innate Immun. 2010;2:406–421. doi: 10.1159/000315469. [DOI] [PubMed] [Google Scholar]
- 22.Yamamoto M, Sato S, Hemmi H, Hoshino K, Kaisho T, Sanjo H, Takeuchi O, Sugiyama M, Okabe M, Takeda K, Akira S. Role of adaptor TRIF in the MyD88-independent toll-like receptor signaling pathway. Science. 2003;301:640–643. doi: 10.1126/science.1087262. [DOI] [PubMed] [Google Scholar]
- 23.Yamamoto M, Takeda K, Akira S. TIR domain-containing adaptors define the specificity of TLR signaling. Mol. Immunol. 2004;40:861–868. doi: 10.1016/j.molimm.2003.10.006. [DOI] [PubMed] [Google Scholar]
- 24.Aloisi F. Immune function of microglia. Glia. 2001;36:165–179. doi: 10.1002/glia.1106. [DOI] [PubMed] [Google Scholar]
- 25.Tambuyzer BR, Bergwerf I, De Vocht N, Reekmans K, Daans J, Jorens PG, Goossens H, Ysebaert DK, Chatterjee S, Van Marck E, Berneman ZN, Ponsaerts P. Allogeneic stromal cell implantation in brain tissue leads to robust microglial activation. Immunol. Cell Biol. 2009;87:268–273. doi: 10.1038/icb.2009.12. [DOI] [PubMed] [Google Scholar]
- 26.Häusler KG, Prinz M, Nolte C, Weber JR, Schumann RR, Kettenmann H, Hanisch UK. Interferon-gamma differentially modulates the release of cytokines and chemokines in lipopolysaccharide- and pneumococcal cell wall-stimulated mouse microglia and macrophages. Eur J Neurosci. 2002;16:2113–2122. doi: 10.1046/j.1460-9568.2002.02287.x. [DOI] [PubMed] [Google Scholar]
- 27.Lehnardt S. Innate immunity and neuroinflammation in the CNS: the role of microglia in Toll-like receptor-mediated neuronal injury. Glia. 2010;58:253–263. doi: 10.1002/glia.20928. [DOI] [PubMed] [Google Scholar]
- 28.Liu X, Chauhan VS, Young AB, Marriott I. NOD2 mediates inflammatory responses of primary murine glia to Streptococcus pneumoniae. Glia. 2010;58:839–847. doi: 10.1002/glia.20968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Chauhan VS, Sterka DG, Jr, Furr SR, Young AB, Marriott I. NOD2 plays an important role in the inflammatory responses of microglia and astrocytes to bacterial CNS pathogens. Glia. 2009;57:414–423. doi: 10.1002/glia.20770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Napoli I, Neumann H. Microglial clearance function in health and disease. Neuroscience. 2009;158:1030–1038. doi: 10.1016/j.neuroscience.2008.06.046. [DOI] [PubMed] [Google Scholar]
- 31.Rotshenker S. The role of Galectin-3/MAC-2 in the activation of the innate-immune function of phagocytosis in microglia in injury and disease. J. Mol. Neurosci. 2009;39:99–103. doi: 10.1007/s12031-009-9186-7. [DOI] [PubMed] [Google Scholar]
- 32.Kielian T, Mayes P, Kielian M. Characterization of microglial responses to Staphylococcus aureus: effects on cytokine, costimulatory molecule, and Toll-like receptor expression. J. Neuroimmunol. 2002;130:86–99. doi: 10.1016/s0165-5728(02)00216-3. [DOI] [PubMed] [Google Scholar]
- 33.Laflamme N, Echchannaoui H, Landmann R, Rivest S. Cooperation between toll-like receptor 2 and 4 in the brain of mice challenged with cell wall components derived from gram-negative and gram-positive bacteria. Eur J Immunol. 2003;33:1127–1138. doi: 10.1002/eji.200323821. [DOI] [PubMed] [Google Scholar]
- 34.Zekki H, Feinstein DL, Rivest S. The clinical course of experimental autoimmune encephalomyelitis is associated with a profound and sustained transcriptional activation of the genes encoding toll-like receptor 2 and CD14 in the mouse CNS. Brain Pathol. 2002;12:308–319. doi: 10.1111/j.1750-3639.2002.tb00445.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Olson JK, Miller SD. Microglia initiate central nervous system innate and adaptive immune responses through multiple TLRs. J Immunol. 2004;173:3916–3924. doi: 10.4049/jimmunol.173.6.3916. [DOI] [PubMed] [Google Scholar]
- 36.Rasley A, Anguita J, Marriott I. Borrelia burgdorferi induces inflammatory mediator production by murine microglia. J Neuroimmunol. 2002;130:22–31. doi: 10.1016/s0165-5728(02)00187-x. [DOI] [PubMed] [Google Scholar]
- 37.Kielian T, Esen N, Bearden ED. Toll-like receptor 2 (TLR2) is pivotal for recognition of S. aureus peptidoglycan but not intact bacteria by microglia. Glia. 2005;49:567–576. doi: 10.1002/glia.20144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Bsibsi M, Ravid R, Gveric D, van Noort JM. Broad expression of Toll-like receptors in the human central nervous system. J. Neuropathol. Exp. Neurol. 2002;61:1013–1021. doi: 10.1093/jnen/61.11.1013. [DOI] [PubMed] [Google Scholar]
- 39.Laflamme N, Soucy G, Rivest S. Circulating cell wall components derived from gram-negative, not gram-positive, bacteria cause a profound induction of the gene-encoding Toll-like receptor 2 in the CNS. J. Neurochem. 2001;79:648–657. doi: 10.1046/j.1471-4159.2001.00603.x. [DOI] [PubMed] [Google Scholar]
- 40.Ebert S, Gerber J, Bader S, Muhlhauser F, Brechtel K, Mitchell TJ, Nau R. Dose-dependent activation of microglial cells by Toll-like receptor agonists alone and in combination. J Neuroimmunol. 2005;159:87–96. doi: 10.1016/j.jneuroim.2004.10.005. [DOI] [PubMed] [Google Scholar]
- 41.Chien HF, Yeh KY, Jiang-Shieh YF, Wei IH, Chang CY, Chang ML, Wu CH. Signal transduction pathways of nitric oxide release in primary microglial culture challenged with gram-positive bacterial constituent, lipoteichoic acid. Neuroscience. 2005;133:423–436. doi: 10.1016/j.neuroscience.2004.09.067. [DOI] [PubMed] [Google Scholar]
- 42.Jung DY, Lee H, Jung BY, Ock J, Lee MS, Lee WH, Suk K. TLR4, but not TLR2, signals autoregulatory apoptosis of cultured microglia: a critical role of IFN-beta as a decision maker. J Immunol. 2005;174:6467–6476. doi: 10.4049/jimmunol.174.10.6467. [DOI] [PubMed] [Google Scholar]
- 43.Laflamme N, Rivest S. Toll-like receptor 4: the missing link of the cerebral innate immune response triggered by circulating gram-negative bacterial cell wall components. Faseb J. 2001;15:155–163. doi: 10.1096/fj.00-0339com. [DOI] [PubMed] [Google Scholar]
- 44.Chakravarty S, Herkenham M. Toll-like receptor 4 on nonhematopoietic cells sustains CNS inflammation during endotoxemia, independent of systemic cytokines. J Neurosci. 2005;25:1788–1796. doi: 10.1523/JNEUROSCI.4268-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Rivest S. Molecular insights on the cerebral innate immune system. Brain Behav Immun. 2003;17:13–19. doi: 10.1016/s0889-1591(02)00055-7. [DOI] [PubMed] [Google Scholar]
- 46.Lehnardt S, Lachance C, Patrizi S, Lefebvre S, Follett PL, Jensen FE, Rosenberg PA, Volpe JJ, Vartanian T. The toll-like receptor TLR4 is necessary for lipopolysaccharide-induced oligodendrocyte injury in the CNS. J. Neurosci. 2002;22:2478–2486. doi: 10.1523/JNEUROSCI.22-07-02478.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kitamura Y, Kakimura J, Koike H, Umeki M, Gebicke-Haerter PJ, Nomura Y, Taniguchi T. Effects of 15-deoxy-delta(12,14) prostaglandin J(2) and interleukin-4 in Toll-like receptor-4-mutant glial cells. Eur J Pharmacol. 2001;411:223–230. doi: 10.1016/s0014-2999(00)00910-9. [DOI] [PubMed] [Google Scholar]
- 48.Qin L, Li G, Qian X, Liu Y, Wu X, Liu B, Hong JS, Block ML. Interactive role of the toll-like receptor 4 and reactive oxygen species in LPS-induced microglia activation. Glia. 2005;52:78–84. doi: 10.1002/glia.20225. [DOI] [PubMed] [Google Scholar]
- 49.Takeshita S, Takeshita F, Haddad DE, Janabi N, Klinman DM. Activation of microglia and astrocytes by CpG oligodeoxynucleotides. Neuroreport. 2001;12:3029–3032. doi: 10.1097/00001756-200110080-00010. [DOI] [PubMed] [Google Scholar]
- 50.Zhang Z, Guo K, Schluesener HJ. The immunostimulatory activity of CpG oligonucleotides on microglial N9 cells is affected by a polyguanosine motif. J Neuroimmunol. 2005;161:68–77. doi: 10.1016/j.jneuroim.2004.12.009. [DOI] [PubMed] [Google Scholar]
- 51.Iliev AI, Stringaris AK, Nau R, Neumann H. Neuronal injury mediated via stimulation of microglial toll-like receptor-9 (TLR9) Faseb J. 2004;18:412–414. doi: 10.1096/fj.03-0670fje. [DOI] [PubMed] [Google Scholar]
- 52.Dalpke AH, Schafer MK, Frey M, Zimmermann S, Tebbe J, Weihe E, Heeg K. Immunostimulatory CpG-DNA activates murine microglia. J Immunol. 2002;168:4854–4863. doi: 10.4049/jimmunol.168.10.4854. [DOI] [PubMed] [Google Scholar]
- 53.Lehnardt S, Massillon L, Follett P, Jensen FE, Ratan R, Rosenberg PA, Volpe JJ, Vartanian T. Activation of innate immunity in the CNS triggers neurodegeneration through a Toll-like receptor 4-dependent pathway. Proc Natl Acad Sci U S A. 2003;100:8514–8519. doi: 10.1073/pnas.1432609100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Diebold SS, Kaisho T, Hemmi H, Akira S, Reis e Sousa C. Innate antiviral responses by means of TLR7-mediated recognition of single-stranded RNA. Science. 2004;303:1529–1531. doi: 10.1126/science.1093616. [DOI] [PubMed] [Google Scholar]
- 55.Heil F, Hemmi H, Hochrein H, Ampenberger F, Kirschning C, Akira S, Lipford G, Wagner H, Bauer S. Species-specific recognition of single-stranded RNA via toll-like receptor 7 and 8. Science. 2004;303:1526–1529. doi: 10.1126/science.1093620. [DOI] [PubMed] [Google Scholar]
- 56.Hemmi H, Kaisho T, Takeuchi O, Sato S, Sanjo H, Hoshino K, Horiuchi T, Tomizawa H, Takeda K, Akira S. Small anti-viral compounds activate immune cells via the TLR7 MyD88-dependent signaling pathway. Nat Immunol. 2002;3:196–200. doi: 10.1038/ni758. [DOI] [PubMed] [Google Scholar]
- 57.Butchi NB, Du M, Peterson KE. Interactions between TLR7 and TLR9 agonists and receptors regulate innate immune responses by astrocytes and microglia. Glia. 2010;58:650–664. doi: 10.1002/glia.20952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Hasan U, Chaffois C, Gaillard C, Saulnier V, Merck E, Tancredi S, Guiet C, Briere F, Vlach J, Lebecque S, Trinchieri G, Bates EE. Human TLR10 is a functional receptor, expressed by B cells and plasmacytoid dendritic cells, which activates gene transcription through MyD88. J Immunol. 2005;174:2942–2950. doi: 10.4049/jimmunol.174.5.2942. [DOI] [PubMed] [Google Scholar]
- 59.Mishra BB, Gundra UM, Teale JM. Expression and distribution of Toll-like receptors 11–13 in the brain during murine neurocysticercosis. J. Neuroinflammation. 2008;5 doi: 10.1186/1742-2094-5-53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Farina C, Aloisi F, Meinl E. Astrocytes are active players in cerebral innate immunity. Trends Immunol. 2007;28:138–145. doi: 10.1016/j.it.2007.01.005. [DOI] [PubMed] [Google Scholar]
- 61.Bowman CC, Rasley A, Tranguch SL, Marriott I. Cultured astrocytes express toll-like receptors for bacterial products. Glia. 2003;43:281–291. doi: 10.1002/glia.10256. [DOI] [PubMed] [Google Scholar]
- 62.Carpentier PA, Begolka WS, Olson JK, Elhofy A, Karpus WJ, Miller SD. Differential activation of astrocytes by innate and adaptive immune stimuli. Glia. 2005;49:360–374. doi: 10.1002/glia.20117. [DOI] [PubMed] [Google Scholar]
- 63.Esen N, Tanga FY, DeLeo JA, Kielian T. Toll-like receptor 2 (TLR2) mediates astrocyte activation in response to the Gram-positive bacterium Staphylococcus aureus. J. Neurochem. 2004;88:746–758. doi: 10.1046/j.1471-4159.2003.02202.x. [DOI] [PubMed] [Google Scholar]
- 64.Henn A, Kirner S, Leist M. TLR2 Hypersensitivity of Astrocytes as Functional Consequence of Previous Inflammatory Episodes. J. Immunol. 2011;186:3237–3247. doi: 10.4049/jimmunol.1002787. [DOI] [PubMed] [Google Scholar]
- 65.Scumpia PO, Kelly KM, Reeves WH, Stevens BR. Double-stranded RNA signals antiviral and inflammatory programs and dysfunctional glutamate transport in TLR3-expressing astrocytes. Glia. 2005 doi: 10.1002/glia.20234. [DOI] [PubMed] [Google Scholar]
- 66.Bsibsi M, Persoon-Deen C, Verwer RW, Meeuwsen S, Ravid R, Van Noort JM. Toll-like receptor 3 on adult human astrocytes triggers production of neuroprotective mediators. Glia. 2006;53:688–695. doi: 10.1002/glia.20328. [DOI] [PubMed] [Google Scholar]
- 67.Gorina R, Font-Nieves M, Márquez-Kisinousky L, Santalucia T, Planas AM. Astrocyte TLR4 activation induces a proinflammatory environment through the interplay between MyD88-dependent NFκB signaling, MAPK, and Jak1/Stat1 pathways. Glia. 2011;59:242–255. doi: 10.1002/glia.21094. [DOI] [PubMed] [Google Scholar]
- 68.Saura J. Microglial cells in astroglial cultures: a cautionary note. J. Neuroinflammation. 2007;4 doi: 10.1186/1742-2094-4-26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Hosoi T, Suzuki S, Nomura J, Ono A, Okuma Y, Akira S, Nomura Y. Bacterial DNA induced iNOS expression through MyD88-p38 MAP kinase in mouse primary cultured glial cells. Brain Res Mol Brain Res. 2004;124:159–164. doi: 10.1016/j.molbrainres.2004.02.014. [DOI] [PubMed] [Google Scholar]
- 70.Lee S, Hong J, Choi SY, Oh SB, Park K, Kim JS, Karin M, Lee SJ. CpG oligodeoxynucleotides induce expression of proinflammatory cytokines and chemokines in astrocytes: the role of c-Jun N-terminal kinase in CpG ODN-mediated NF-kappaB activation. J Neuroimmunol. 2004;153:50–63. doi: 10.1016/j.jneuroim.2004.04.013. [DOI] [PubMed] [Google Scholar]
- 71.Okun E, Griffioen KJ, Lathia JD, Tang SC, Mattson MP, Arumugam TV. Toll-like receptors in neurodegeneration. Brain Res. Rev. 2009;59:278–292. doi: 10.1016/j.brainresrev.2008.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.van Noort JM, Bsibsi M. Toll-like receptors in the CNS: implications for neurodegeneration and repair. Prog. Brain Res. 2009;175:139–148. doi: 10.1016/S0079-6123(09)17509-X. [DOI] [PubMed] [Google Scholar]
- 73.Setzu A, Lathia JD, Zhao C, Wells K, Rao MS, Ffrench-Constant C, Franklin RJ. Inflammation stimulates myelination by transplanted oligodendrocyte precursor cells. Glia. 2006;54:297–303. doi: 10.1002/glia.20371. [DOI] [PubMed] [Google Scholar]
- 74.Kigerl KA, Lai W, Rivest S, Hart RP, Satoskar AR, Popovich PG. Toll-like receptor (TLR)-2 and TLR-4 regulate inflammation, gliosis, and myelin sparing after spinal cord injury. Neurochem. 2007;102:37–50. doi: 10.1111/j.1471-4159.2007.04524.x. [DOI] [PubMed] [Google Scholar]
- 75.Schonberg DL, Popovich PG, McTigue DM. Oligodendrocyte generation is differentially influenced by toll-like receptor (TLR) 2 and TLR4-mediated intraspinal macrophage activation. J. Neuropathol. Exp. Neurol. 2007;66:1124–1135. doi: 10.1097/nen.0b013e31815c2530. [DOI] [PubMed] [Google Scholar]
- 76.Tang SC, Arumugam TV, Xu X, Cheng A, Mughal MR, Jo DG, Lathia JD, Siler DA, Chigurupati S, Ouyang X, Magnus T, Camandola S, Mattson MP. Pivotal role for neuronal Toll-like receptors in ischemic brain injury and functional deficits. Proc. Natl. Acad. Sci. U.S.A. 2007;104:13798–13803. doi: 10.1073/pnas.0702553104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Mishra BB, Mishra PK, Teale JM. Expression and distribution of Toll-like receptors in the brain during murine neurocysticercosis. J. Neuroimmunol. 2006;181:46–56. doi: 10.1016/j.jneuroim.2006.07.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Wadachi R, Hargreaves KM. Trigeminal nociceptors express TLR-4 and CD14: a mechanism for pain due to infection. J. Dent. Res. 2006;85:49–53. doi: 10.1177/154405910608500108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Préhaud C, Mégret F, Lafage M, Lafon M. Virus infection switches TLR-3-positive human neurons to become strong producers of beta interferon. J. Virol. 2005;79:12893–12904. doi: 10.1128/JVI.79.20.12893-12904.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Acosta C, Davies A. Bacterial lipopolysaccharide regulates nociceptin expression in sensory neurons. J. Neurosci. Res. 2008;86:1077–1086. doi: 10.1002/jnr.21565. [DOI] [PubMed] [Google Scholar]
- 81.Divanovic S, Trompette A, Atabani SF, Madan R, Golenbock DT, Visintin A, Finberg RW, Tarakhovsky A, Vogel SN, Belkaid Y, Kurt-Jones EA, Karp CL. Inhibition of TLR-4/MD-2 signaling by RP105/MD-1. J. Endotoxin Res. 2005;11:363–368. doi: 10.1179/096805105X67300. [DOI] [PubMed] [Google Scholar]
- 82.Lathia JD, Okun E, Tang SC, Griffioen K, Cheng A, Mughal MR, Laryea G, Selvaraj PK, ffrench-Constant C, Magnus T, Arumugam TV, Mattson MP. Toll-like receptor 3 is a negative regulator of embryonic neural progenitor cell proliferation. J. Neurosci. 2008;28:13978–13984. doi: 10.1523/JNEUROSCI.2140-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Okun E, Griffioen KJ, Son TG, Lee JH, Roberts NJ, Mughal MR, Hutchison E, Cheng A, Arumugam TV, Lathia JD, van Praag H, Mattson MP. TLR2 activation inhibits embryonic neural progenitor cell proliferation. J. Neurochem. 2010;114:462–474. doi: 10.1111/j.1471-4159.2010.06778.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Ma Y, Li J, Chiu I, Wang Y, Sloane JA, Lü J, Kosaras B, Sidman RL, Volpe JJ, Vartanian T. Toll-like receptor 8 functions as a negative regulator of neurite outgrowth and inducer of neuronal apoptosis. J. Cell Biol. 2006;175:209–215. doi: 10.1083/jcb.200606016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Rolls A, Shechter R, London A, Ziv Y, Ronen A, Levy R, Schwartz M. Toll-like receptors modulate adult hippocampal neurogenesis. Nat. Cell Biol. 2007;9:1081–1088. doi: 10.1038/ncb1629. [DOI] [PubMed] [Google Scholar]
- 86.van de Beek D, de Gans J, Spanjaard L, Weisfelt M, Reitsma JB, Vermeulen M. Clinical features and prognostic factors in adults with bacterial meningitis. N. Engl. J. Med. 2004;351:1849–1859. doi: 10.1056/NEJMoa040845. [DOI] [PubMed] [Google Scholar]
- 87.Koedel U. Toll-like receptors in bacterial meningitis. Curr. Top. Microbiol. Immunol. 2009;336 doi: 10.1007/978-3-642-00549-7_2. [DOI] [PubMed] [Google Scholar]
- 88.Tuomanen E, Liu H, Hengstler B, Zak O, Tomasz A. The induction of meningeal inflammation by components of the pneumococcal cell wall. J. Infect. Dis. 1985;151:859–868. doi: 10.1093/infdis/151.5.859. [DOI] [PubMed] [Google Scholar]
- 89.Koedel U, Scheld WM, Pfister HW. Pathogenesis and pathophysiology of pneumococcal meningitis. Lancet Infect. Dis. 2002;2:721–736. doi: 10.1016/s1473-3099(02)00450-4. [DOI] [PubMed] [Google Scholar]
- 90.Weber JR, Tuomanen EI. Cellular damage in bacterial meningitis: an interplay of bacterial and host driven toxicity. J. Neuroimmunol. 2007;184:45–52. doi: 10.1016/j.jneuroim.2006.11.016. [DOI] [PubMed] [Google Scholar]
- 91.Yoshimura A, Lien E, Ingalls RR, Tuomanen E, Dziarski R, Golenbock D. Cutting edge: recognition of Gram-positive bacterial cell wall components by the innate immune system occurs via Toll-like receptor 2. J. Immunol. 1999;163:1–5. [PubMed] [Google Scholar]
- 92.Dziarski R, Wang Q, Miyake K, Kirschning CJ, Gupta D. MD-2 enables Toll-like receptor 2 (TLR2)-mediated responses to lipopolysaccharide and enhances TLR2-mediated responses to Gram-positive and Gram-negative bacteria and their cell wall components. J. Immunol. 2001;166:1938–1944. doi: 10.4049/jimmunol.166.3.1938. [DOI] [PubMed] [Google Scholar]
- 93.Takeuchi O, Hoshino K, Kawai T, Sanjo H, Takada H, Ogawa T, Takeda K, Akira S. Differential roles of TLR2 and TLR4 in recognition of gram-negative and gram-positive bacterial cell wall components. Immunity. 1999;11:443–451. doi: 10.1016/s1074-7613(00)80119-3. [DOI] [PubMed] [Google Scholar]
- 94.Malley R, Henneke P, Morse SC, Cieslewicz MJ, Lipsitch M, Thompson CM, Kurt-Jones E, Paton JC, Wessels MR, Golenbock DT. Recognition of pneumolysin by Toll-like receptor 4 confers resistance to pneumococcal infection. Proc. Natl. Acad. Sci. U S A. 2003;100:1966–1971. doi: 10.1073/pnas.0435928100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Mogensen TH, Paludan SR, Kilian M, Ostergaard L. Live Streptococcus pneumoniae, Haemophilus influenzae, and Neisseria meningitidis activate the inflammatory response through Toll-like receptors 2, 4, and 9 in species-specific patterns. J. Leukoc. Biol. 2006;80:267–277. doi: 10.1189/jlb.1105626. [DOI] [PubMed] [Google Scholar]
- 96.Schmeck B, Huber S, Moog K, Zahlten J, Hocke AC, Opitz B, Hammerschmidt S, Mitchell TJ, Kracht M, Rosseau S, Suttorp N, Hippenstiel S. Pneumococci induced TLR- and Rac1-dependent NF-kappaB-recruitment to the IL-8 promoter in lung epithelial cells. Am. J. Physiol. Lung Cell. Mol. Physiol. 2006;290:L730–L737. doi: 10.1152/ajplung.00271.2005. [DOI] [PubMed] [Google Scholar]
- 97.Lee KS, Scanga CA, Bachelder EM, Chen Q, Snapper CM. TLR2 synergizes with both TLR4 and TLR9 for induction of the MyD88-dependent splenic cytokine and chemokine response to Streptococcus pneumoniae. Cell Immunol. 2007;245:103–110. doi: 10.1016/j.cellimm.2007.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Klein M, Obermaier B, Angele B, Pfister HW, Wagner H, Koedel U, Kirschning CJ. Innate immunity to pneumococcal infection of the central nervous system depends on toll-like receptor (TLR) 2 and TLR4. J. Infect. Dis. 2008;198:1028–1036. doi: 10.1086/591626. [DOI] [PubMed] [Google Scholar]
- 99.Flo TH, Halaas O, Lien E, Ryan L, Teti G, Golenbock DT, Sundan A, Espevik T. Human toll-like receptor 2 mediates monocyte activation by Listeria monocytogenes, but not by group B streptococci or lipopolysaccharide. J. Immunol. 2000;164:2064–2069. doi: 10.4049/jimmunol.164.4.2064. [DOI] [PubMed] [Google Scholar]
- 100.Seki E, Tsutsui H, Tsuji NM, Hayashi N, Adachi K, Nakano H, Futatsugi-Yumikura S, Takeuchi O, Hoshino K, Akira S, Fujimoto J, Nakanishi K. Critical roles of myeloid differentiation factor 88-dependent proinflammatory cytokine release in early phase clearance of Listeria monocytogenes in mice. J. Immunol. 2002;169:3863–3868. doi: 10.4049/jimmunol.169.7.3863. [DOI] [PubMed] [Google Scholar]
- 101.Janot L, Secher T, Torres D, Maillet I, Pfeilschifter J, Quesniaux VF, Landmann R, Ryffel B, Erard F. CD14 works with toll-like receptor 2 to contribute to recognition and control of Listeria monocytogenes infection. J. Infect. Dis. 2008;198:115–124. doi: 10.1086/588815. [DOI] [PubMed] [Google Scholar]
- 102.Hayashi F, Smith KD, Ozinsky A, Hawn TR, Yi EC, Goodlett DR, Eng JK, Akira S, Underhill DM, Aderem A. The innate immune response to bacterial flagellin is mediated by Toll-like receptor 5. Nature. 2001;410:1099–1103. doi: 10.1038/35074106. [DOI] [PubMed] [Google Scholar]
- 103.Echchannaoui H, Frei K, Schnell C, Leib SL, Zimmerli W, Landmann R. Toll-like receptor 2-deficient mice are highly susceptible to Streptococcus pneumoniae meningitis because of reduced bacterial clearing and enhanced inflammation. J. Infect. Dis. 2002;186:798–806. doi: 10.1086/342845. [DOI] [PubMed] [Google Scholar]
- 104.Koedel U, Angele B, Rupprecht T, Wagner H, Roggenkamp A, Pfister HW, Kirschning CJ. Toll-like receptor 2 participates in mediation of immune response in experimental pneumococcal meningitis. J. Immunol. 2003;170:438–444. doi: 10.4049/jimmunol.170.1.438. [DOI] [PubMed] [Google Scholar]
- 105.Koedel U, Rupprecht T, Angele B, Heesemann J, Wagner H, Pfister HW, Kirschning CJ. MyD88 is required for mounting a robust host immune response to Streptococcus pneumoniae in the CNS. Brain. 2004;127:1437–1445. doi: 10.1093/brain/awh171. [DOI] [PubMed] [Google Scholar]
- 106.Zwijnenburg PJ, van der Poll T, Florquin S, Roord JJ, Van Furth AM. IL-1 receptor type 1 gene-deficient mice demonstrate an impaired host defense against pneumococcal meningitis. J. Immunol. 2003;170:4724–4730. doi: 10.4049/jimmunol.170.9.4724. [DOI] [PubMed] [Google Scholar]
- 107.Zwijnenburg PJ, van der Poll T, Florquin S, Akira S, Takeda K, Roord JJ, van Furth AM. Interleukin-18 gene-deficient mice show enhanced defense and reduced inflammation during pneumococcal meningitis. J. Neuroimmunol. 2003;138:31–37. doi: 10.1016/s0165-5728(03)00088-2. [DOI] [PubMed] [Google Scholar]
- 108.Liu S, Kielian T. Microglial activation by Citrobacter koseri is mediated by TLR4- and MyD88-dependent pathways. J Immunol. 2009;183:5537–5547. doi: 10.4049/jimmunol.0900083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Garg S, Nichols JR, Esen N, Liu S, Phulwani NK, Syed MM, Wood WH, Zhang Y, Becker KG, Aldrich A, Kielian T. MyD88 expression by CNS-resident cells is pivotal for eliciting protective immunity in brain abscesses. ASN Neuro. 2009;1:pii: e00007. doi: 10.1042/AN20090004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Kielian T. Immunopathogenesis of brain abscess. J. Neuroinflammation. 2004;1 doi: 10.1186/1742-2094-1-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Mathisen GE, Johnson JP. Brain abscess. Clin. Infect. Dis. 1997;25:763–779. doi: 10.1086/515541. [DOI] [PubMed] [Google Scholar]
- 112.Jones ME, Draghi DC, Karlowsky JA, Sahm DF, Bradley JS. Prevalence of antimicrobial resistance in bacteria isolated from central nervous system specimens as reported by U.S. hospital laboratories from 2000 to 2002. Ann. Clin. Microbiol. Antimicrob. 2004;3 doi: 10.1186/1476-0711-3-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Prasad KN, Mishra AM, Gupta D, Husain N, Husain M, Gupta RK. Analysis of microbial etiology and mortality in patients with brain abscess. J. Infect. 2006;53:221–227. doi: 10.1016/j.jinf.2005.12.002. [DOI] [PubMed] [Google Scholar]
- 114.Greenberg BM. Central nervous system infections in the intensive care unit. Semin. Neurol. 2008;28:682–689. doi: 10.1055/s-0028-1105976. [DOI] [PubMed] [Google Scholar]
- 115.Tenney JH. Bacterial infections of the central nervous system in neurosurgery. Neurol. Clin. 1986;4:91–114. [PubMed] [Google Scholar]
- 116.Schliamser SE, Bäckman K, Norrby SR. Intracranial abscesses in adults: an analysis of 54 consecutive cases. Scand. J. Infect. Dis. 1988;20:1–9. doi: 10.3109/00365548809117210. [DOI] [PubMed] [Google Scholar]
- 117.Townsend GC, Scheld WM. Infections of the central nervous system. Adv. Intern. Med. 1998;43:403–447. [PubMed] [Google Scholar]
- 118.Smith NP, Nelson MR, Moore D, Gazzard BG. Cerebral abscesses in a patient with AIDS caused by methicillin-resistant Staphylococcus aureus (MRSA) Int. J. STD AIDS. 1997;8:459–460. doi: 10.1258/0956462971920352. [DOI] [PubMed] [Google Scholar]
- 119.Khan MA, Greig JR, Jayamohan J. Community-acquired methicillin-resistant Staphylococcus aureus brain abscess in an immunocompetent individual. Scand. J. Infect. Dis. 2000;32:423–424. doi: 10.1080/003655400750045042. [DOI] [PubMed] [Google Scholar]
- 120.Roche M, Humphreys H, Smyth E, Phillips J, Cunney R, McNamara E, O'Brien D, McArdle O. A twelve-year review of central nervous system bacterial abscesses; presentation and aetiology. Clin. Microbiol. Infect. 2003;9:803–809. doi: 10.1046/j.1469-0691.2003.00651.x. [DOI] [PubMed] [Google Scholar]
- 121.Naesens R, Ronsyn M, Druwe P, Denis O, Ieven M, Jeurissen A. Central nervous system invasion by community-acquired meticillin-resistant Staphylococcus aureus. J. Med. Microbiol. 2009;58:1247–1251. doi: 10.1099/jmm.0.011130-0. [DOI] [PubMed] [Google Scholar]
- 122.Kielian T, Haney A, Mayes PM, Garg S, Esen N. Toll-like receptor 2 modulates the proinflammatory milieu in Staphylococcus aureus-induced brain abscess. Infect. Immun. 2005;73:7428–7435. doi: 10.1128/IAI.73.11.7428-7435.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Esen N, Kielian T. Central role for MyD88 in the responses of microglia to pathogen-associated molecular patterns. J. Immunol. 2006;176:6802–6811. doi: 10.4049/jimmunol.176.11.6802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Kielian T, Phulwani NK, Esen N, Syed MM, Haney AC, McCastlain K, Johnson J. MyD88-dependent signals are essential for the host immune response in experimental brain abscess. J. Immunol. 2007;178:4528–4537. doi: 10.4049/jimmunol.178.7.4528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Nichols JR, Aldrich AL, Mariani MM, Vidlak D, Esen N, Kielian T. TLR2 deficiency leads to increased Th17 infiltrates in experimental brain abscesses. J. Immunol. 2009;182:7119–7130. doi: 10.4049/jimmunol.0802656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Vidlak D, Mariani MM, Aldrich A, Liu S, Kielian T. Roles of Toll-like receptor 2 (TLR2) and superantigens on adaptive immune responses during CNS staphylococcal infection. Brain Behav. Immun. 2010 doi: 10.1016/j.bbi.2010.09.016. PMID: 20868736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Stenzel W, Soltek S, Sanchez-Ruiz M, Akira S, Miletic H, Schlüter D, Deckert M. Both TLR2 and TLR4 are required for the effective immune response in Staphylococcus aureus-induced experimental murine brain abscess. Am. J. Pathol. 2008;172:132–145. doi: 10.2353/ajpath.2008.070567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Cua DJ, Tato CM. Innate IL-17-producing cells: the sentinels of the immune system. Nat. Rev. Immunol. 2010;10:479–489. doi: 10.1038/nri2800. [DOI] [PubMed] [Google Scholar]
- 129.Witowski J, Pawlaczyk K, Breborowicz A, Scheuren A, Kuzlan-Pawlaczyk M, Wisniewska J, Polubinska A, Friess H, Gahl GM, Frei U, Jorres A. IL-17 stimulates intraperitoneal neutrophil infiltration through the release of GRO alpha chemokine from mesothelial cells. J. Immunol. 2000;165:5814–5821. doi: 10.4049/jimmunol.165.10.5814. [DOI] [PubMed] [Google Scholar]
- 130.Kielian T, Barry B, Hickey WF. CXC chemokine receptor-2 ligands are required for neutrophil-mediated host defense in experimental brain abscesses. J. Immunol. 2001;166:4634–4643. doi: 10.4049/jimmunol.166.7.4634. [DOI] [PubMed] [Google Scholar]
- 131.Sifri CD, Park J, Helm GA, Stemper ME, Shukla SK. Fatal brain abscess due to community-associated methicillin-resistant Staphylococcus aureus strain USA300. Clin. Infect. Dis. 2007;45:e113–e117. doi: 10.1086/522171. [DOI] [PubMed] [Google Scholar]
- 132.Kielian T, Bearden ED, Baldwin AC, Esen N. IL-1 and TNF-alpha play a pivotal role in the host immune response in a mouse model of Staphylococcus aureus-induced experimental brain abscess. J. Neuropathol. Exp. Neurol. 2004;63:381–396. doi: 10.1093/jnen/63.4.381. [DOI] [PubMed] [Google Scholar]
- 133.Kawai T, Akira S. Antiviral signaling through pattern recognition receptors. J. Biochem. 2007;141:137–145. doi: 10.1093/jb/mvm032. [DOI] [PubMed] [Google Scholar]
- 134.Kawai T, Akira S. The role of pattern-recognition receptors in innate immunity: update on Toll-like receptors. Nat. Immunol. 2010;11:373–384. doi: 10.1038/ni.1863. [DOI] [PubMed] [Google Scholar]
- 135.Hoffman RW, Gazitt T, Foecking MF, Ortmann RA, Misfeldt M, Jorgenson R, Young SL, Greidinger EL. U1 RNA induces innate immunity signaling. Arthritis Rheum. 2004;50:2891–2896. doi: 10.1002/art.20428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Fairhurst AM, Hwang SH, Wang A, Tian XH, Boudreaux C, Zhou XJ, Casco J, Li QZ, Connolly JE, Wakeland EK. Yaa autoimmune phenotypes are conferred by overexpression of TLR7. Eur. J. Immunol. 2008;38:1971–1978. doi: 10.1002/eji.200838138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.O'Neill LA, Bowie AG. The family of five: TIR-domain-containing adaptors in Toll-like receptor signalling. Nat. Rev. Immunol. 2007;7:353–364. doi: 10.1038/nri2079. [DOI] [PubMed] [Google Scholar]
- 138.Looker KJ, Garnett GP. A systematic review of the epidemiology and interaction of herpes simplex virus types 1 and 2. Sex. Transm. Infect. 2005;81:103–107. doi: 10.1136/sti.2004.012039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Kimberlin DW. Herpes simplex virus infections of the central nervous system. Semin. Pediatr. Infect. Dis. 2003;14:83–89. doi: 10.1053/spid.2003.127224. [DOI] [PubMed] [Google Scholar]
- 140.Romero JR, Kimberlin DW. Molecular diagnosis of viral infections of the central nervous system. Clin. Lab. Med. 2003;23:843–865. doi: 10.1016/s0272-2712(03)00084-2. [DOI] [PubMed] [Google Scholar]
- 141.Tyler KL. Herpes simplex virus infections of the central nervous system: encephalitis and meningitis, including Mollaret's. Herpes. 2004;11:57A–64A. [PubMed] [Google Scholar]
- 142.Kimberlin DW. Management of HSV encephalitis in adults and neonates: diagnosis, prognosis and treatment. Herpes. 2007;14:11–16. [PubMed] [Google Scholar]
- 143.Padgett DA, Sheridan JF, Dorne J, Berntson GG, Candelora J, Glaser R. Social stress and the reactivation of latent herpes simplex virus type 1. Proc. Natl. Acad. Sci. U.S.A. 1998;95:7231–7235. doi: 10.1073/pnas.95.12.7231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Goade DE, Nofchissey RA, Kusewitt DF, Hjelle B, Kreisel J, Moore J, Lyons CR. Ultraviolet light induces reactivation in a murine model of cutaneous herpes simplex virus-1 infection. Photochem. Photobiol. 2001;74:108–114. doi: 10.1562/0031-8655(2001)074<0108:uliria>2.0.co;2. [DOI] [PubMed] [Google Scholar]
- 145.Ichihashi M, Nagai H, Matsunaga K. Sunlight is an important causative factor of recurrent herpes simplex. Cutis. 2004;74:14–18. [PubMed] [Google Scholar]
- 146.Schiff D, Rosenblum MK. Herpes simplex encephalitis (HSE) and the immunocompromised: a clinical and autopsy study of HSE in the settings of cancer and human immunodeficiency virus-type 1 infection. Hum. Pathol. 1998;29:215–222. doi: 10.1016/s0046-8177(98)90038-7. [DOI] [PubMed] [Google Scholar]
- 147.Kurt-Jones EA, Belko J, Yu C, Newburger PE, Wang J, Chan M, Knipe DM, Finberg RW. The role of toll-like receptors in herpes simplex infection in neonates. J. Infect. Dis. 2005;191:746–748. doi: 10.1086/427339. [DOI] [PubMed] [Google Scholar]
- 148.Liu X, Fitzgerald K, Kurt-Jones E, Finberg R, Knipe DM. Herpesvirus tegument protein activates NF-kappaB signaling through the TRAF6 adaptor protein. Proc. Natl. Acad. Sci. U.S.A. 2008;105:11335–11339. doi: 10.1073/pnas.0801617105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Kurt-Jones EA, Chan M, Zhou S, Wang J, Reed G, Bronson R, Arnold MM, Knipe DM, Finberg RW. Herpes simplex virus 1 interaction with Toll-like receptor 2 contributes to lethal encephalitis. Proc. Natl. Acad. Sci. U.S.A. 2004;101:1315–1350. doi: 10.1073/pnas.0308057100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Bereczky-Veress B, Abdelmagid N, Piehl F, Bergström T, Olsson T, Sköldenberg B, Diez M. Influence of perineurial cells and Toll-like receptors 2 and 9 on Herpes simplex type 1 entry to the central nervous system in rat encephalitis. PLoS One. 2010;5:e12350. doi: 10.1371/journal.pone.0012350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Sorensen LN, Reinert LS, Malmgaard L, Bartholdy C, Thomsen AR, Paludan SR. TLR2 and TLR9 synergistically control herpes simplex virus infection in the brain. J. Immunol. 2008;181:8604–8612. doi: 10.4049/jimmunol.181.12.8604. [DOI] [PubMed] [Google Scholar]
- 152.Lima GK, Zolini GP, Mansur DS, Freire Lima BH, Wischhoff U, Astigarraga RG, Dias MF, das Graças Almeida Silva M, Béla SR, do Valle Antonelli LR, Arantes RM, Gazzinelli RT, Báfica A, Kroon EG, Campos MA. Toll-like receptor (TLR) 2 and TLR9 expressed in trigeminal ganglia are critical to viral control during herpes simplex virus 1 infection. Am. J. Pathol. 2010;177:2433–2445. doi: 10.2353/ajpath.2010.100121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Wang T, Town T, Alexopoulou L, Anderson JF, Fikrig E, Flavell RA. Toll-like receptor 3 mediates West Nile virus entry into the brain causing lethal encephalitis. Nat. Med. 2004;10:1366–1373. doi: 10.1038/nm1140. [DOI] [PubMed] [Google Scholar]
- 154.Daffis S, Samuel MA, Suthar MS, Gale M, Jr, Diamond MS. Toll-like receptor 3 has a protective role against West Nile virus infection. J. Virol. 2008;82:10349–10358. doi: 10.1128/JVI.00935-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Szretter KJ, Daffis S, Patel J, Suthar MS, Klein RS, Gale M, Jr, Diamond MS. The innate immune adaptor molecule MyD88 restricts West Nile virus replication and spread in neurons of the central nervous system. J. Virol. 2010;84:12125–12138. doi: 10.1128/JVI.01026-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Szretter KJ, Samuel MA, Gilfillan S, Fuchs A, Colonna M, Diamond MS. The immune adaptor molecule SARM modulates tumor necrosis factor alpha production and microglia activation in the brainstem and restricts West Nile Virus pathogenesis. J. Virol. 2009;83:9329–9338. doi: 10.1128/JVI.00836-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Mishra BB, Gundra UM, Teale JM. Toll-like receptors in CNS parasitic infections. Curr. Top. Microbiol. Immunol. 2009;336 doi: 10.1007/978-3-642-00549-7_5. [DOI] [PubMed] [Google Scholar]
- 158.Scanga CA, Aliberti J, Jankovic D, Tilloy F, Bennouna S, Denkers EY, Medzhitov R, Sher A. Cutting edge: MyD88 is required for resistance to Toxoplasma gondii infection and regulates parasite-induced IL-12 production by dendritic cells. J. Immunol. 2002;168:5997–6001. doi: 10.4049/jimmunol.168.12.5997. [DOI] [PubMed] [Google Scholar]
- 159.Drennan MB, Stijlemans B, Van den Abbeele J, Quesniaux VJ, Barkhuizen M, Brombacher F, De Baetselier P, Ryffel B, Magez S. The induction of a type 1 immune response following a Trypanosoma brucei infection is MyD88 dependent. J. Immunol. 2005;175:2501–2509. doi: 10.4049/jimmunol.175.4.2501. [DOI] [PubMed] [Google Scholar]
- 160.Gillette-Ferguson I, Hisem AG, Sun Y, Diaconu E, McGarry HF, Taylor MJ, Pearlman E. Wolbachia- and Onchocerca volvulus-induced keratitis (river blindness) is dependent on myeloid differentiation factor 88. Infect. Immun. 2006;74:2442–2445. doi: 10.1128/IAI.74.4.2442-2445.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Mishra BB, Gundra UM, Wong K, Teale JM. MyD88-deficient mice exhibit decreased parasite-induced immune responses but reduced disease severity in a murine model of neurocysticercosis. Infect. Immun. 2009;77:5369–5379. doi: 10.1128/IAI.00455-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Lepenies B, Cramer JP, Burchard GD, Wagner H, Kirschning CJ, Jacobs T. Induction of experimental cerebral malaria is independent of TLR2/4/9. Med. Microbiol. Immunol. 2008;197:39–44. doi: 10.1007/s00430-007-0057-y. [DOI] [PubMed] [Google Scholar]
- 163.Togbe D, Schofield L, Grau GE, Schnyder B, Boissay V, Charron S, Rose S, Beutler B, Quesniaux VF, Ryffel B. Murine cerebral malaria development is independent of toll-like receptor signalingMurine cerebral malaria development is independent of toll-like receptor signaling. Am. J. Pathol. 2007;170:1640–1648. doi: 10.2353/ajpath.2007.060889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Coban C, Ishii KJ, Uematsu S, Arisue N, Sato S, Yamamoto M, Kawai T, Takeuchi O, Hisaeda H, Horii T, Akira S. Pathological role of Toll-like receptor signaling in cerebral malaria. Int Immunol. 2007;19:67–79. doi: 10.1093/intimm/dxl123. [DOI] [PubMed] [Google Scholar]
- 165.Griffith JW, O'Connor C, Bernard K, Town T, Goldstein DR, Bucala R. Toll-like receptor modulation of murine cerebral malaria is dependent on the genetic background of the host. J. Infect. Dis. 2007;196:1553–1564. doi: 10.1086/522865. [DOI] [PubMed] [Google Scholar]
- 166.Béjot Y, Osseby GV, Gremeaux V, Durier J, Rouaud O, Moreau T, Giroud M. Changes in risk factors and preventive treatments by stroke subtypes over 20 years: a population-based study. J. Neurol. Sci. 2009;287:84–88. doi: 10.1016/j.jns.2009.08.062. [DOI] [PubMed] [Google Scholar]
- 167.Nishino H, Aihara N, Czurko A, Hashitani T, Isobe Y, Ichikawa O, Watari H. Reconstruction of GABAergic transmission and behavior by striatal cell grafts in rats with ischemic infarcts in the middle cerebral artery. J. Neural Transplant. Plast. 1993;4:147–155. doi: 10.1155/NP.1993.147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Globus MY, Busto R, Martinez E, Valdés I, Dietrich WD. Ischemia induces release of glutamate in regions spared from histopathologic damage in the rat. Stroke. 1990;21:III43–6. [PubMed] [Google Scholar]
- 169.Jayaraman T, Paget A, Shin YS, Li X, Mayer J, Chaudhry H, Niimi Y, Silane M, Berenstein A. TNF-alpha-mediated inflammation in cerebral aneurysms: a potential link to growth and rupture. Vasc. Health Risk. Manag. 2008;4:805–817. doi: 10.2147/vhrm.s2700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Kilic U, Kilic E, Matter CM, Bassetti CL, Hermann DM. TLR-4 deficiency protects against focal cerebral ischemia and axotomy-induced neurodegeneration. Neurobiol. Dis. 2008;31:33–40. doi: 10.1016/j.nbd.2008.03.002. [DOI] [PubMed] [Google Scholar]
- 171.Zhou ML, Shi JX, Hang CH, Zhang FF, Gao J, Yin HX. Expression of Toll-like receptor 4 in the brain in a rabbit experimental subarachnoid haemorrhage model. Inflamm. Res. 2007;56:93–97. doi: 10.1007/s00011-006-6035-9. [DOI] [PubMed] [Google Scholar]
- 172.Lehnardt S, Lehmann S, Kaul D, Tschimmel K, Hoffmann O, Cho S, Krueger C, Nitsch R, Meisel A, Weber JR. Toll-like receptor 2 mediates CNS injury in focal cerebral ischemia. J. Neuroimmunol. 2007;190:28–33. doi: 10.1016/j.jneuroim.2007.07.023. [DOI] [PubMed] [Google Scholar]
- 173.Ziegler G, Harhausen D, Schepers C, Hoffmann O, Röhr C, Prinz V, König J, Lehrach H, Nietfeld W, Trendelenburg G. TLR2 has a detrimental role in mouse transient focal cerebral ischemia. Biochem. Biophys. Res. Commun. 2007;359:574–579. doi: 10.1016/j.bbrc.2007.05.157. [DOI] [PubMed] [Google Scholar]
- 174.Hyakkoku K, Hamanaka J, Tsuruma K, Shimazawa M, Tanaka H, Uematsu S, Akira S, Inagaki N, Nagai H, Hara H. Toll-like receptor 4 (TLR4), but not TLR3 or TLR9, knock-out mice have neuroprotective effects against focal cerebral ischemia. Neuroscience. 2010;171:258–267. doi: 10.1016/j.neuroscience.2010.08.054. [DOI] [PubMed] [Google Scholar]
- 175.Famakin B, Mou Y, Ruetzler C, Joliet B, Dragan M, Hallenbeck J. Disruption of downstream MyD88 or TRIF Toll-like receptor Signaling does not protect against cerebral ischemia. Brain Res. 2011 doi: 10.1016/j.brainres.2011.02.074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Letiembre M, Liu Y, Walter S, Hao W, Pfander T, Wrede A, Schulz-Schaeffer W, Fassbender K. Screening of innate immune receptors in neurodegenerative diseases: a similar pattern. Neurobiol. Aging. 2009;30:759–768. doi: 10.1016/j.neurobiolaging.2007.08.018. [DOI] [PubMed] [Google Scholar]
- 177.Letiembre M, Hao W, Liu Y, Walter S, Mihaljevic I, Rivest S, Hartmann T, Fassbender K. Innate immune receptor expression in normal brain aging. Neuroscience. 2007;146:248–254. doi: 10.1016/j.neuroscience.2007.01.004. [DOI] [PubMed] [Google Scholar]
- 178.Miller KR, Streit WJ. The effects of aging, injury and disease on microglial function: a case for cellular senescence. Neuron Glia Biol. 2007;3:245–253. doi: 10.1017/S1740925X08000136. [DOI] [PubMed] [Google Scholar]
- 179.Weldon DT, Rogers SD, Ghilardi JR, Finke MP, Cleary JP, O'Hare E, Esler WP, Maggio JE, Mantyh PW. Fibrillar beta-amyloid induces microglial phagocytosis, expression of inducible nitric oxide synthase, and loss of a select population of neurons in the rat CNS in vivo. J. Neurosci. 1998;18:2161–2173. doi: 10.1523/JNEUROSCI.18-06-02161.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Kopec KK, Carroll RT. Alzheimer's beta-amyloid peptide 1–42 induces a phagocytic response in murine microglia. J. Neurochem. 1998;71:2123–2131. doi: 10.1046/j.1471-4159.1998.71052123.x. [DOI] [PubMed] [Google Scholar]
- 181.Akiyama H, Barger S, Barnum S, Bradt B, Bauer J, Cole GM, Cooper NR, Eikelenboom P, Emmerling M, Fiebich BL, Finch CE, Frautschy S, Griffin WS, Hampel H, Hull M, Landreth G, Lue L, Mrak R, Mackenzie IR, McGeer PL, O'Banion MK, Pachter J, Pasinetti G, Plata-Salaman C, Rogers J, Rydel R, Shen Y, Streit W, Strohmeyer R, Tooyoma I, Van Muiswinkel FL, Veerhuis R, Walker D, Webster S, Wegrzyniak B, Wenk G, Wyss-Coray T. Inflammation and Alzheimer's disease. Neurobiol. Aging. 2000;21:383–421. doi: 10.1016/s0197-4580(00)00124-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Bornemann KD, Wiederhold KH, Pauli C, Ermini F, Stalder M, Schnell L, Sommer B, Jucker M, Staufenbiel M. Abeta-induced inflammatory processes in microglia cells of APP23 transgenic mice. Am. J. Pathol. 2001;158:63–73. doi: 10.1016/s0002-9440(10)63945-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Bolmont T, Haiss F, Eicke D, Radde R, Mathis CA, Klunk WE, Kohsaka S, Jucker M, Calhoun ME. Dynamics of the microglial/amyloid interaction indicate a role in plaque maintenance. J. Neurosci. 2008;28:4283–4292. doi: 10.1523/JNEUROSCI.4814-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Bamberger ME, Harris ME, McDonald DR, Husemann J, Landreth GE. A cell surface receptor complex for fibrillar beta-amyloid mediates microglial activation. J. Neurosci. 2003;23:2665–2674. doi: 10.1523/JNEUROSCI.23-07-02665.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Reed-Geaghan EG, Savage JC, Hise AG, Landreth GE. CD14 and toll-like receptors 2 and 4 are required for fibrillar A{beta}-stimulated microglial activation. J. Neurosci. 2009;29:11982–11992. doi: 10.1523/JNEUROSCI.3158-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Tükel C, Wilson RP, Nishimori JH, Pezeshki M, Chromy BA, Bäumler AJ. Responses to amyloids of microbial and host origin are mediated through toll-like receptor 2. Cell Host Microbe. 2009;6:45–53. doi: 10.1016/j.chom.2009.05.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Tahara K, Kim HD, Jin JJ, Maxwell JA, Li L, Fukuchi K. Role of toll-like receptor signalling in Abeta uptake and clearance. Brain. 2006;129:3006–3019. doi: 10.1093/brain/awl249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Frank S, Copanaki E, Burbach GJ, Müller UC, Deller T. Differential regulation of toll-like receptor mRNAs in amyloid plaque-associated brain tissue of aged APP23 transgenic mice. Neurosci. Lett. 2009;453:41–44. doi: 10.1016/j.neulet.2009.01.075. [DOI] [PubMed] [Google Scholar]
- 189.Udan ML, Ajit D, Crouse NR, Nichols MR. Toll-like receptors 2 and 4 mediate Abeta(1–42) activation of the innate immune response in a human monocytic cell line. J. Neurochem. 2008;104:524–533. doi: 10.1111/j.1471-4159.2007.05001.x. [DOI] [PubMed] [Google Scholar]
- 190.Stewart CR, Stuart LM, Wilkinson K, van Gils JM, Deng J, Halle A, Rayner KJ, Boyer L, Zhong R, Frazier WA, Lacy-Hulbert A, El Khoury J, Golenbock DT, Moore KJ. CD36 ligands promote sterile inflammation through assembly of a Toll-like receptor 4 and 6 heterodimer. Nat. Immunol. 2010;11:155–161. doi: 10.1038/ni.1836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Jin JJ, Kim HD, Maxwell JA, Li L, Fukuchi K. Toll-like receptor 4-dependent upregulation of cytokines in a transgenic mouse model of Alzheimer's disease. J. Neuroinflammation. 2008;5 doi: 10.1186/1742-2094-5-23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Richard KL, Filali M, Prefontaine P, Rivest S. Toll-like receptor 2 acts as a natural innate immune receptor to clear amyloid beta 1–42 and delay the cognitive decline in a mouse model of Alzheimer's disease. J. Neurosci. 2008;28:5784–5793. doi: 10.1523/JNEUROSCI.1146-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Tang SC, Lathia JD, Selvaraj PK, Jo DG, Mughal MR, Cheng A, Siler DA, Markesbery WR, Arumugam TV, Mattson MP. Toll-like receptor-4 mediates neuronal apoptosis induced by amyloid beta-peptide and the membrane lipid peroxidation product 4-hydroxynonenal. Exp. Neurol. 2008;213:114–121. doi: 10.1016/j.expneurol.2008.05.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Scholtzova H, Kascsak RJ, Bates KA, Boutajangout A, Kerr DJ, Meeker HC, Mehta PD, Spinner DS, Wisniewski T. Induction of toll-like receptor 9 signaling as a method for ameliorating Alzheimer's disease-related pathology. J. Neurosci. 2009;29:1846–1854. doi: 10.1523/JNEUROSCI.5715-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Vollmar P, Kullmann JS, Thilo B, Claussen MC, Rothhammer V, Jacobi H, Sellner J, Nessler S, Korn T, Hemmer B. Active immunization with amyloid-beta 1–42 impairs memory performance through TLR2/4-dependent activation of the innate immune system. J. Immunol. 2010;185:6338–6347. doi: 10.4049/jimmunol.1001765. [DOI] [PubMed] [Google Scholar]
- 196.Lublin FD. Delayed, relapsing experimental allergic encephalomyelitis in mice. Role of adjuvants and pertussis vaccine. J Neurol Sci. 1982;57:105–110. doi: 10.1016/0022-510x(82)90114-9. [DOI] [PubMed] [Google Scholar]
- 197.Brown AM, McFarlin DE. Relapsing experimental allergic encephalomyelitis in the SJL/J mouse. Lab Invest. 1981;45:278–284. [PubMed] [Google Scholar]
- 198.Hansen BS, Hussain RZ, Lovett-Racke AE, Thomas JA, Racke MK. Multiple toll-like receptor agonists act as potent adjuvants in the induction of autoimmunity. J Neuroimmunol. 2006;172:94–103. doi: 10.1016/j.jneuroim.2005.11.006. [DOI] [PubMed] [Google Scholar]
- 199.Segal BM, Chang JT, Shevach EM. CpG oligonucleotides are potent adjuvants for the activation of autoreactive encephalitogenic T cells in vivo. J Immunol. 2000;164:5683–5688. doi: 10.4049/jimmunol.164.11.5683. [DOI] [PubMed] [Google Scholar]
- 200.Deng C, Radu C, Diab A, Tsen MF, Hussain R, Cowdery JS, Racke MK, Thomas JA. IL-1 receptor-associated kinase 1 regulates susceptibility to organ-specific autoimmunity. J Immunol. 2003;170:2833–2842. doi: 10.4049/jimmunol.170.6.2833. [DOI] [PubMed] [Google Scholar]
- 201.Visser L, Jan de Heer H, Boven LA, van Riel D, van Meurs M, Melief MJ, Zahringer U, van Strijp J, Lambrecht BN, Nieuwenhuis EE, Laman JD. Proinflammatory bacterial peptidoglycan as a cofactor for the development of central nervous system autoimmune disease. J Immunol. 2005;174:808–816. doi: 10.4049/jimmunol.174.2.808. [DOI] [PubMed] [Google Scholar]
- 202.Prinz M, Garbe F, Schmidt H, Mildner A, Gutcher I, Wolter K, Piesche M, Schroers R, Weiss E, Kirschning CJ, Rochford CD, Brück W, Becher B. Innate immunity mediated by TLR9 modulates pathogenicity in an animal model of multiple sclerosis. J. Clin. Invest. 2006;116:456–464. doi: 10.1172/JCI26078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Cohen SJ, Cohen IR, Nussbaum G. IL-10 mediates resistance to adoptive transfer experimental autoimmune encephalomyelitis in MyD88(−/−) mice. J. Immunol. 2010;184:212–221. doi: 10.4049/jimmunol.0900296. [DOI] [PubMed] [Google Scholar]
- 204.Marta M, Andersson A, Isaksson M, Kämpe O, Lobell A. Unexpected regulatory roles of TLR4 and TLR9 in experimental autoimmune encephalomyelitis. Eur. J. Immunol. 2008;38:565–575. doi: 10.1002/eji.200737187. [DOI] [PubMed] [Google Scholar]
- 205.National Spinal Cord Injury Statistical Center. 2010 Feb; doi: 10.1080/10790268.2002.11753637. [DOI] [PubMed] [Google Scholar]
- 206.Beers MH, Kaplan JL, editors. The Merck Manual of Diagnosis and Therapy. Merck Sharp & Dohme Corp; 2006. [Google Scholar]
- 207.Jackson AB, Dijkers M, DeVivo MJ, Poczatek RB. A demographic profile of new traumatic spinal cord injuries: change and stability over 30 years. Arch. Phys. Med. Rehabil. 2004;85:1740–1748. doi: 10.1016/j.apmr.2004.04.035. [DOI] [PubMed] [Google Scholar]
- 208.Prasad D, Schiff D. Malignant spinal-cord compression. Lancet Oncol. 2005;6:15–24. doi: 10.1016/S1470-2045(04)01709-7. [DOI] [PubMed] [Google Scholar]
- 209.Shedid D, Benzel EC. Cervical spondylosis anatomy: pathophysiology and biomechanics. Neurosurgery. 2007;60:S7–S13. doi: 10.1227/01.NEU.0000215430.86569.C4. [DOI] [PubMed] [Google Scholar]
- 210.Babb A, Carlson WO. Vertebral compression fractures: treatment and evaluation. S D Med. 2006;59:343–345. [PubMed] [Google Scholar]
- 211.Dusart I, Schwab ME. Secondary cell death and the inflammatory reaction after dorsal hemisection of the rat spinal cord. Eur. J. Neurosci. 1994;6:712–724. doi: 10.1111/j.1460-9568.1994.tb00983.x. [DOI] [PubMed] [Google Scholar]
- 212.Fehlings MG, Sekhon LH. Acute interventions in spinal cord injury: what do we know, what should we do? Clin. Neurosurg. 2001;48:226–242. [PubMed] [Google Scholar]
- 213.Fitch MT, Doller C, Combs CK, Landreth GE, Silver J. Cellular and molecular mechanisms of glial scarring and progressive cavitation: in vivo and in vitro analysis of inflammation-induced secondary injury after CNS trauma. J. Neurosci. 1999;19:8182–8198. doi: 10.1523/JNEUROSCI.19-19-08182.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214.Popovich PG, Guan Z, McGaughy V, Fisher L, Hickey WF, Basso DM. The neuropathological and behavioral consequences of intraspinal microglial/macrophage activation. J. Neuropathol. Exp. Neurol. 2002;61:623–633. doi: 10.1093/jnen/61.7.623. [DOI] [PubMed] [Google Scholar]
- 215.Su Y, Zhang Z, Trautmann K, Xu S, Schluesener HJ. TLR and NOD2 ligands induce cell proliferation in the rat intact spinal cord. J. Neuropathol. Exp. Neurol. 2005;64:991–997. doi: 10.1097/01.jnen.0000187051.74265.56. [DOI] [PubMed] [Google Scholar]
- 216.Zhang Z, Trautmann K, Schluesener HJ. Microglia activation in rat spinal cord by systemic injection of TLR3 and TLR7/8 agonists. J. Neuroimmunol. 2005;164:154–160. doi: 10.1016/j.jneuroim.2005.03.014. [DOI] [PubMed] [Google Scholar]
- 217.Thuret S, Moon LD, Gage FH. Therapeutic interventions after spinal cord injury. Nat. Rev. Neurosci. 2006;7:628–643. doi: 10.1038/nrn1955. [DOI] [PubMed] [Google Scholar]
- 218.Bradbury EJ, McMahon SB. Spinal cord repair strategies: why do they work? Nat. Rev. Neurosci. 2006;7:644–653. doi: 10.1038/nrn1964. [DOI] [PubMed] [Google Scholar]
- 219.Hanisch UK, Johnson TV, Kipnis J. Toll-like receptors: roles in neuroprotection? Trends Neurosci. 2008;31:176–182. doi: 10.1016/j.tins.2008.01.005. [DOI] [PubMed] [Google Scholar]
- 220.Okun E, Griffioen KJ, Mattson MP. Toll-like receptor signaling in neural plasticity and disease. Trends Neurosci. 2011;34:269–268. doi: 10.1016/j.tins.2011.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221.Cunningham C, Campion S, Lunnon K, Murray CL, Woods JF, Deacon RM, Rawlins JN, Perry VH. Systemic inflammation induces acute behavioral and cognitive changes and accelerates neurodegenerative disease. Biol. Psychiatry. 2009;65:304–312. doi: 10.1016/j.biopsych.2008.07.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222.Okun E, Griffioen K, Barak B, Roberts NJ, Castro K, Pita MA, Cheng A, Mughal MR, Wan R, Ashery U, Mattson MP. Toll-like receptor 3 inhibits memory retention and constrains adult hippocampal neurogenesis. Proc. Natl. Acad. Sci. U.S.A. 2010;107:15625–15630. doi: 10.1073/pnas.1005807107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223.Ji JF, He BP, Dheen ST, Tay SS. Expression of chemokine receptors CXCR4, CCR2, CCR5 and CX3CR1 in neural progenitor cells isolated from the subventricular zone of the adult rat brain. Neurosci. Lett. 2004;355:236–240. doi: 10.1016/j.neulet.2003.11.024. [DOI] [PubMed] [Google Scholar]
- 224.Ni HT, Hu S, Sheng WS, Olson JM, Cheeran MC, Chan AS, Lokensgard JR, Peterson PK. High-level expression of functional chemokine receptor CXCR4 on human neural precursor cells. Brain Res. Dev. Brain Res. 2004;152:159–169. doi: 10.1016/j.devbrainres.2004.06.015. [DOI] [PubMed] [Google Scholar]
- 225.Kim BW, Son H. Neural cell adhesion molecule (NCAM) induces neuronal phenotype acquisition in dominant negative MEK1-expressing hippocampal neural progenitor cells. Exp. Mol. Med. 2006;38:732–738. doi: 10.1038/emm.2006.86. [DOI] [PubMed] [Google Scholar]
- 226.Keohane A, Ryan S, Maloney E, Sullivan AM, Nolan YM. Tumour necrosis factor-alpha impairs neuronal differentiation but not proliferation of hippocampal neural precursor cells: Role of Hes1. Mol. Cell Neurosci. 2010;43:127–135. doi: 10.1016/j.mcn.2009.10.003. [DOI] [PubMed] [Google Scholar]
- 227.Ben-Hur T, Ben-Menachem O, Furer V, Einstein O, Mizrachi-Kol R, Grigoriadis N. Effects of proinflammatory cytokines on the growth, fate, and motility of multipotential neural precursor cells. Mol. Cell Neurosci. 2003;24:623–631. doi: 10.1016/s1044-7431(03)00218-5. [DOI] [PubMed] [Google Scholar]
- 228.Covacu R, Arvidsson L, Andersson A, Khademi M, Erlandsson-Harris H, Harris RA, Svensson MA, Olsson T, Brundin L. TLR activation induces TNF-alpha production from adult neural stem/progenitor cells. J. Immunol. 2009;182:6889–6895. doi: 10.4049/jimmunol.0802907. [DOI] [PubMed] [Google Scholar]
- 229.Mukhopadhyay S, Peiser L, Gordon S. Activation of murine macrophages by Neisseria meningitidis and IFN-gamma in vitro: distinct roles of class A scavenger and Toll-like pattern recognition receptors in selective modulation of surface phenotype. J. Leukoc. Biol. 2004;76:577–584. doi: 10.1189/jlb.0104014. [DOI] [PubMed] [Google Scholar]
- 230.Underhill DM, Gantner B. Integration of Toll-like receptor and phagocytic signaling for tailored immunity. Microbes Infect. 2004;6:1368–1373. doi: 10.1016/j.micinf.2004.08.016. [DOI] [PubMed] [Google Scholar]
- 231.McGettrick AF, O'Neill LA. Localisation and trafficking of Toll-like receptors: an important mode of regulation. Curr. Opin. Immunol. 2010;22:20–27. doi: 10.1016/j.coi.2009.12.002. [DOI] [PubMed] [Google Scholar]
- 232.Barton GM, Kagan JC. A cell biological view of Toll-like receptor function: regulation through compartmentalization. Nat. Rev. Immunol. 2009;9:535–542. doi: 10.1038/nri2587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 233.Baker BJ, Akhtar LN, Benveniste EN. SOCS1 and SOCS3 in the control of CNS immunity. Trends Immunol. 2009;30:392–400. doi: 10.1016/j.it.2009.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 234.Brea D, Blanco M, Ramos-Cabrer P, Moldes O, Arias S, Perez-Mato M, Leira R, Sobrino T, Castillo J. Toll-like receptors 2 and 4 in ischemic stroke: outcome and therapeutic values. J. Cereb. Blood Flow Metab. 2011 doi: 10.1038/jcbfm.2010.231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 235.Downes CE, Crack PJ. Neural injury following stroke: are Toll-like receptors the link between the immune system and the CNS? Br. J. Pharmacol. 2010;160:1872–1888. doi: 10.1111/j.1476-5381.2010.00864.x. [DOI] [PMC free article] [PubMed] [Google Scholar]