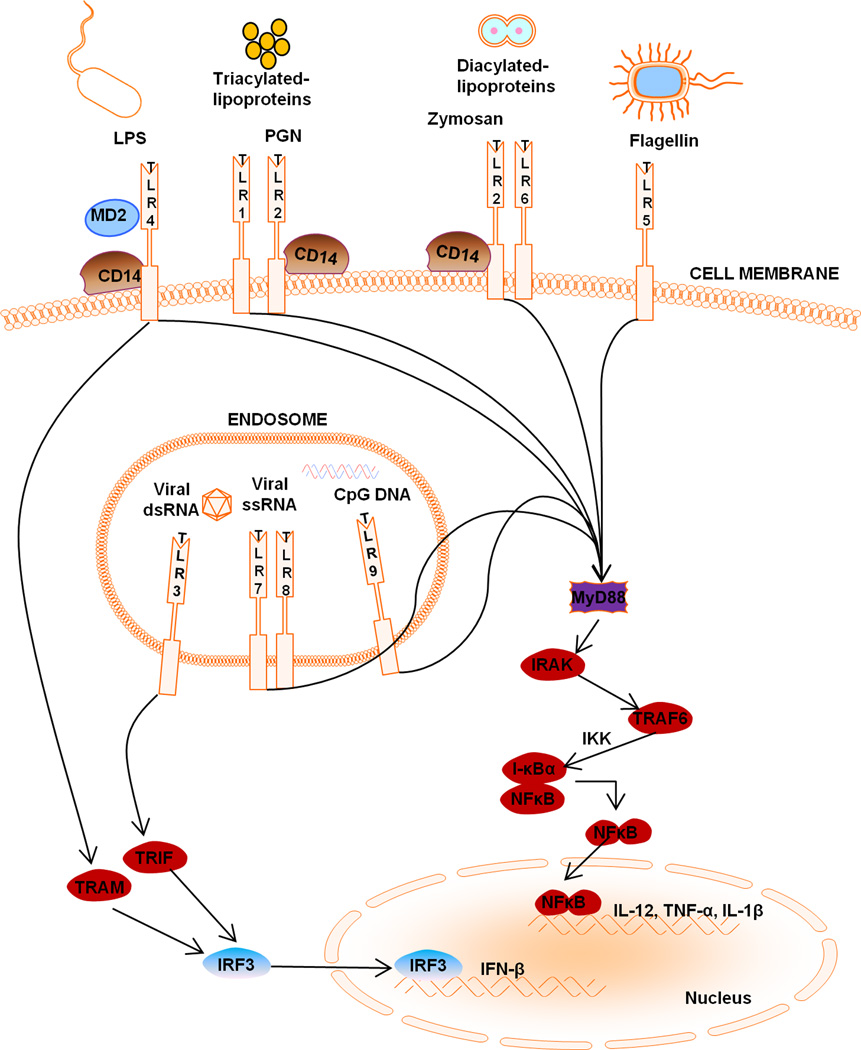
Figure 1. Toll-like receptor (TLR) localization and signaling.
TLR1, TLR2, TLR4, TLR5, and TLR6 are expressed at the cell surface for extracellular ligand recognition whereas TLR3, TLR7, and TLR9 are localized in the endosomal compartment for the recognition of pathogen nucleic acid motifs. All TLRs, with the exception of TLR3, recruit MyD88, while TLR1, TLR2, TLR4 and TLR6 recruit the additional adaptors CD14 and TIRAP, the latter of which links the TIR domain with MyD88. In the MyD88-dependent pathway, MyD88 recruits the IRAK family of proteins which leads to I-κB phosphorylation, resulting in the release and nuclear translocation of NF-κB, which influences the expression of numerous inflammatory genes. TLR3 ligands initiate the TRIF-dependent pathway, whereas TLR4 can signal via either MyD88-dependent or TRIF-dependent pathways requiring the additional linker adaptor TRAM, which links the TIR domain of TLR4 with TRIF. In the TRIF-dependent pathway, TRIF interacts with TRAF3 to activate IRF3 and IRF7 and initiate type I interferon production. Alternatively, TRIF can also bind to RIP1 and TRAF6 and activate NF-κB and MAPK for late-phase (i.e. 24 h) induction of inflammatory gene expression.