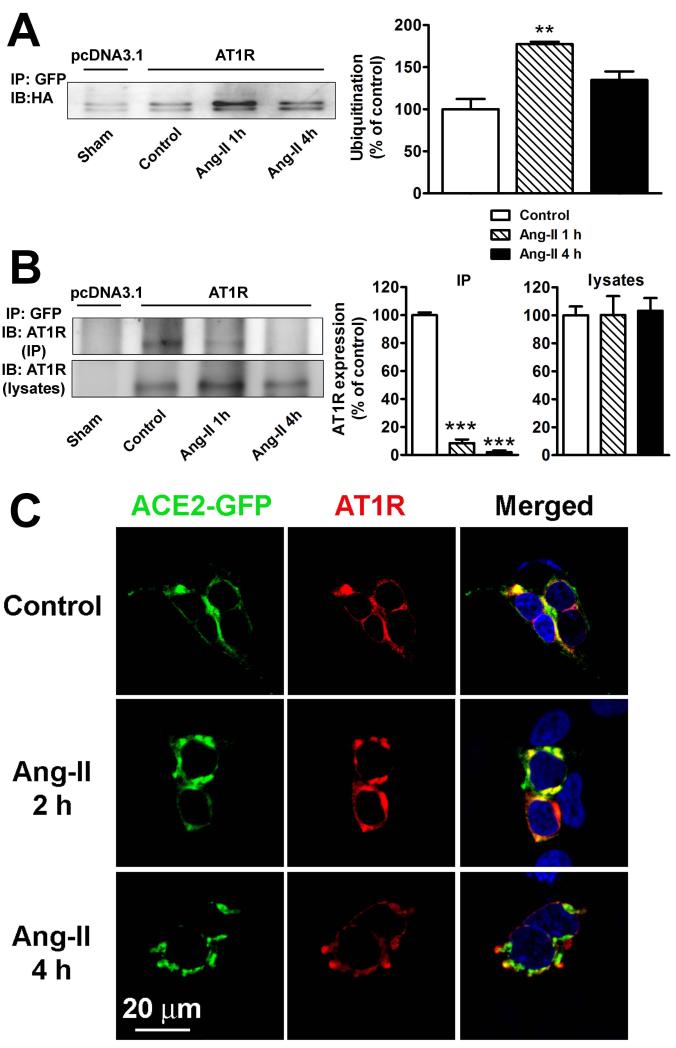
Figure 5.
A. Ang-II stimulates ACE2 ubiquitination. HEK293T cells were transfected in 10 cm2 plates with 10 μg ACE2-GFP, 10 μg HA-ubiquitin, and either 10 μg pcDNA3.1 (1st lane) or 10 μg AT1R (lanes 2-4). Cells were serum starved for 24 h and treated with Ang-II (100 nM) for the indicated time periods. Cells were then lysed and ACE2 interacting proteins were pulled down by treatment with a GFP antibody (2 μg/mg). Cell lysates were separated by 10% SDS-PAGE and ubiquitin levels were revealed by western blotting using anti-HA antibody. The experiment shown is representative from 3 independent transfections. **−indicates P<0.01 B. ACE2 interaction with AT1R in HEK293T cells determined by co-immunoprecipitation. Cells were transfected in 10 cm2 plates with 10 μg ACE2-GFP and 10 μg pcDNA3.1 (lane 1) or 10 μg AT1R (lanes 2-4). Cells were serum starved for 24 hours and treated with Ang-II (100 nM) for the indicated time periods. Following lysis, ACE2 interacting proteins were pulled down by treatment with a GFP antibody (2 μg/mg). AT1R was detected in immunoprecipitates (top panel) and lysates (bottom panel) using an anti-AT1R antibody (SC-579). Similar results were obtained in 2 other experiments. ***−indicates P<0.001 C. ACE2 and AT1R localization in HEK293T cells in control conditions (top panel), or after 2 h (middle panel) and 4 h (bottom panel) treatment with Ang-II (100 nM).