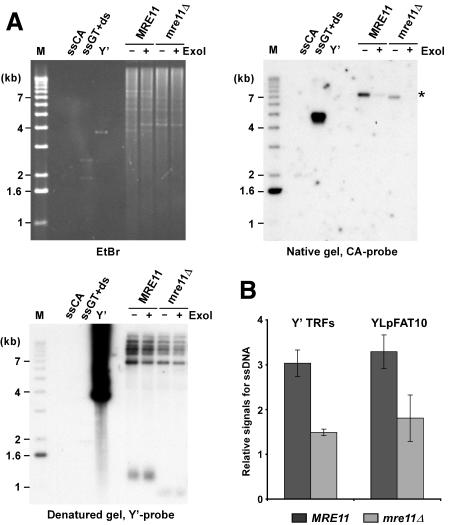
Figure 1.
G-tail signals are reduced in mre11Δ strains. (A) Genomic DNA derived from α-factor-arrested cells was digested with XhoI. G-tails on YLpFAT10 (indicated by asterisk) were analyzed by native in-gel hybridization for wild-type and mre11Δ strains (MLY50 and MLY51). DNAs were either mock treated (labeled ExoI-), or treated with Escherichia coli Exonuclease I (labeled ExoI+), before in-gel analysis. (Top, left) Ethidium-bromide-stained gel. (Top, right) The gel after nondenaturing hybridization to the CA probe. (Bottom, left) The DNA was denatured in the gel, transferred to a nylon membrane, and the membrane hybridized to a Y′ probe. Single-stranded phagemid DNA containing yeast telomeric repeats of the G-rich strand (ssGT) and of the C-rich strand (ssCA) served as positive and negative controls, respectively. The ssGT control was mixed with PvuI-digested pMW55, the latter serving as double-stranded control (ds, a fragment of 1.9 kb). Linearized pVZY′K plasmid (3.9 kb) served as a positive control for the hybridization with the Y′ probe (labeled Y′). (M) End-labeled 1-kb ladder DNA serving as a size standard. (B) Relative amounts for G-tails (relative signals for ssDNA, in arbitrary units) were obtained by calculating the ratio of the respective signals on the native gels over the total amount of Y′ TRF signals or YLpFAT10 signals. Three to six individual nonsynchronous cultures were tested for each strain, and the standard deviation is indicated.