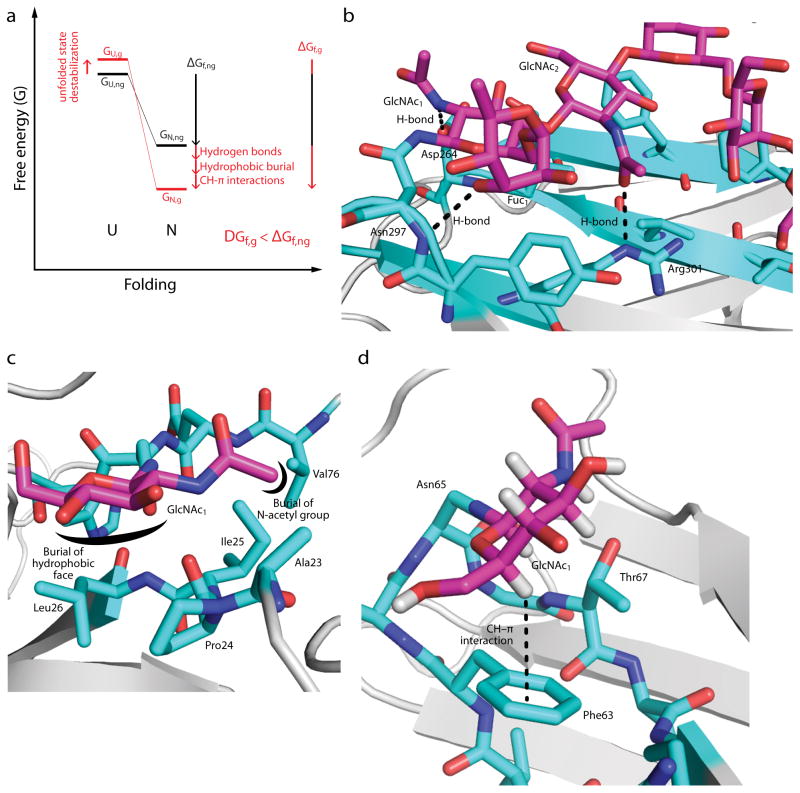
Figure 2. Intrinsic effects of N-glycosylation on protein folding.
(a) A free energy diagram illustrating the change in energy of the unfolded (U) and native (N) states upon N-glycosylation. The energy of the unfolded state of the non-glycosylated protein (GU,ng) tends to increase upon N-glycosylation (GU,g), whereas the energy of the native state of the non-glycosylated protein (GN,ng) tends to decrease (GN,g). The effect of N-glycosylation on the free energy of folding (ΔGf,ng vs. ΔGf,g) is the sum of these effects, and can be on the order of several kcal mol−1 (see text). (b) Glycan-protein H-bonds in the mature, complex-type N-glycan in the Fc fragment of human IgG1 (PDB ID 1FC1). Note that the N-acetyl group of GlcNAc-1 is shown in the energetically unfavorable cis conformation; this may be a mis-assignment, since the electron densities of the acetyl methyl and carbonyl O groups are likely similar at this resolution. (c) Glycan-protein hydrophobic burial in human chorionic gonadotropin (PDB ID: 1HCN). The hydrophobic α-face of GlcNAc-1 is buried in a pocket formed by Pro24, Ile25, Leu26, while the N-acetyl methyl group is buried in an adjacent pocket formed by Ala23, Ile25, and Val76. (d) A glycan–protein CH–π interaction in the adhesion domain of the human protein CD2 (HsCD2ad; PDB ID 1GYA). The hydrogen atom on C5 of GlcNAc-1 interacts with the aromatic side chain of Phe63. The structural module shown is known as an “enhanced aromatic sequon” (see text). Only the first GlcNAc of the glycan is shown for clarity.