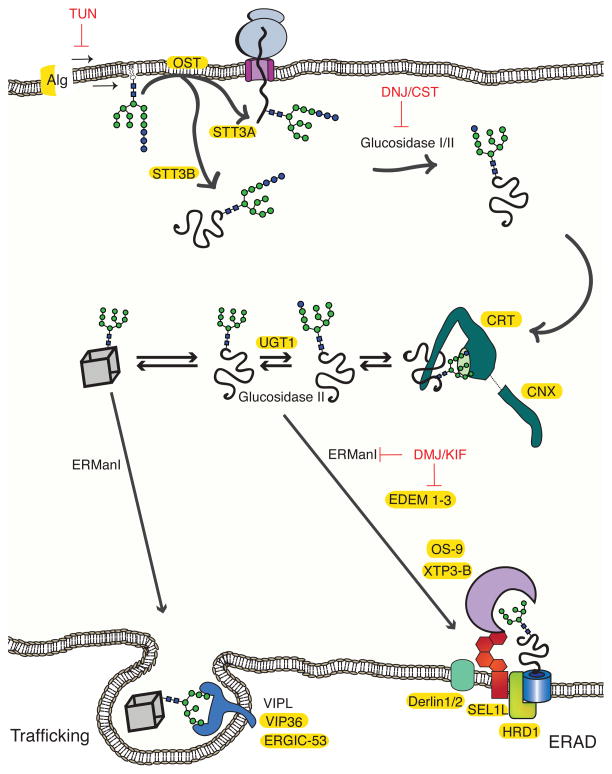
Figure 3. N-linked glycans as protein sorting tags.
N-linked glycans are synthesized by Alg genes and transferred to polypeptides by oligosaccharyltransferase (OST) to asparagine residues in the Asn-Xxx-Ser/Thr sequon. OST exists as two isoforms that differ in their catalytic subunits, STT3A and STT3B. Glucosidase I and II remove the first two glucose moieties (blue spheres), generating a monoglucosylated glycoform, which enables the glycoprotein to enter the lectin folding cycle by engaging calnexin (CNX) and calreticulin (CRT)11. Removal of the remaining glucose by glucosidase II releases the protein from CNX/CRT. If the glycoprotein requires additional folding cycles, a glucose moiety is added by UGT1 and the protein re-engages CNX and CRT. Proteins that have reached their native states (cube) are demannosylated by ER mannosidase I (ERManI). The mannose-trimmed glycans are recognized by ERGIC-53, VIPL and VIP36, which facilitate their packaging into Golgi-bound vesicles52. Proteins that do not fold into their native states undergo extensive mannose trimming by the mannosidase-like proteins EDEM1-3. Glycoforms generated by EDEM1-3 are recognized by the ERAD lectin receptors OS-9 and XTP3-B, which bring the misfolded proteins to the ERAD complex. The relative transcript levels of some of the proteins involved in glycoproteostasis are upregulated by UPR activation (highlighted in yellow)72. A number of enzyme inhibitors (red) have been employed to study the effects of glycan processing on protein trafficking and degradation.