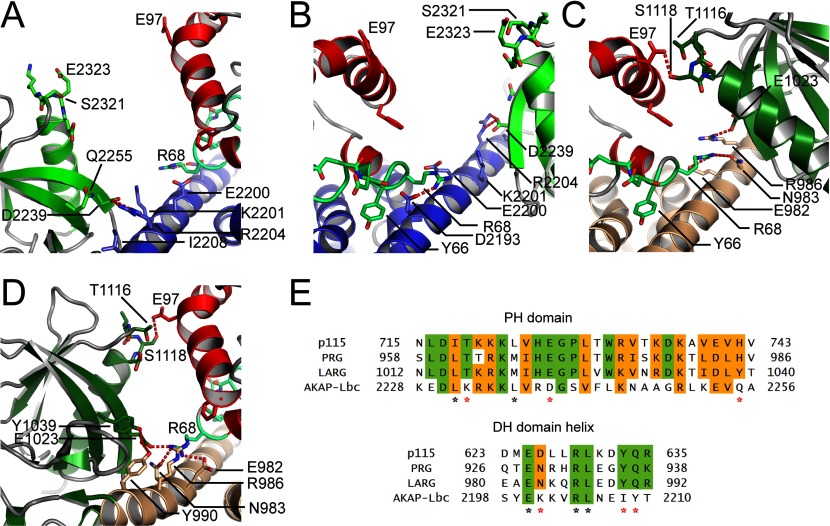
Figure 2. No contact between the AKAP-Lbc PH domain and RhoA.
(A and B) Two views of the separation between RhoA and the PH domain. Residues which in homologous proteins are involved in RhoA–PH domain interactions are shown as a stick representation. RhoA is coloured red, and the AKAP-Lbc DH and PH domains are coloured blue and green. (C and D) Two views, from equivalent orientations as (A) and (B), of the interaction between the PH domain of LARG and RhoA (PDB code 1X86). The LARG DH and PH domains are coloured brown and dark green. (E) Sequence alignment of the regions of the PH domain and DH domain potentially involved in RhoA–PH domain interactions, for AKAP-Lbc and three homologous RhoGEF domains, all three of which show an effect of the PH domain on nucleotide-exchange catalysis. Residues involved in the interface in LARG are indicated with asterisks below the alignment. For residue differences likely to be important for the lack of effect of the AKAP-Lbc PH domain, the asterisks are coloured red.