Fig. 3.
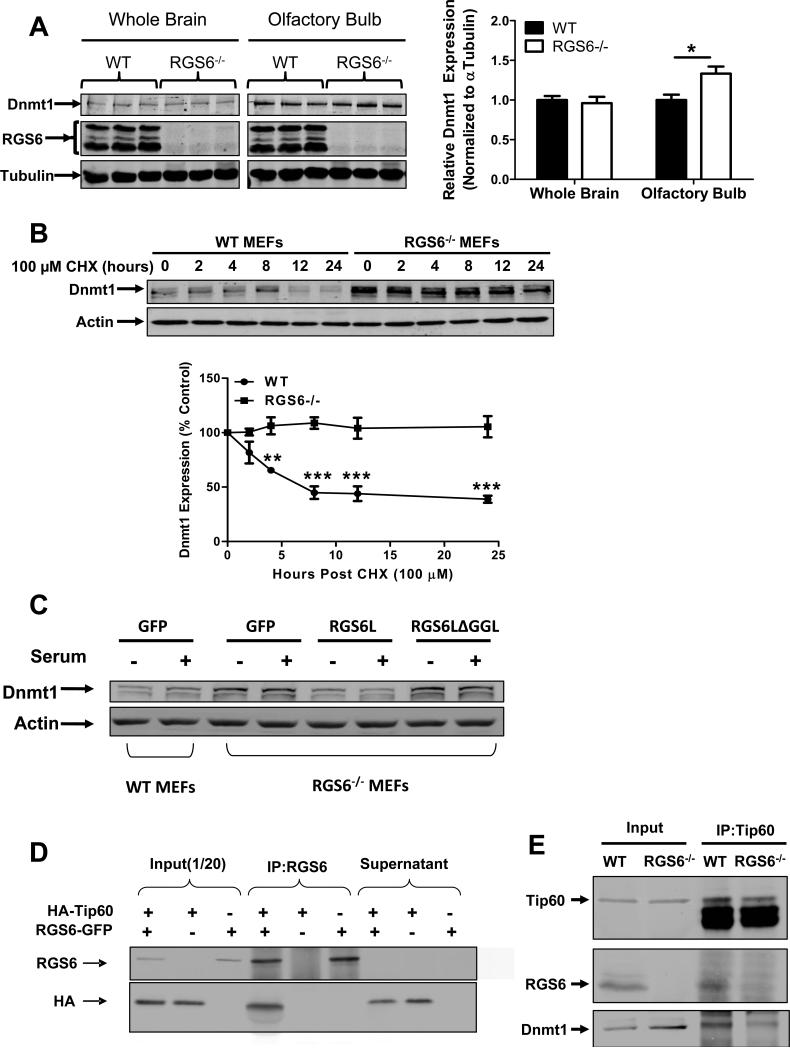
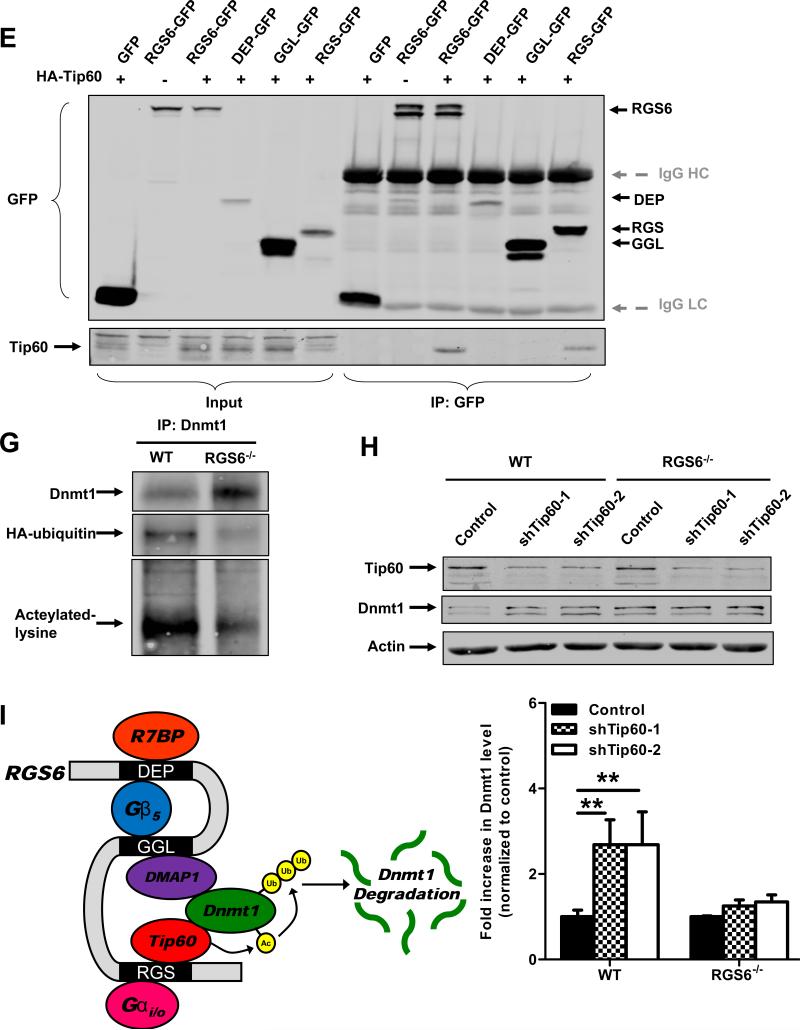
RGS6 promotes degradation of Dnmt1 by scaffolding Dnmt1 and Tip60. (A) Dnmt1 protein levels are increased in RGS6−/− olfactory bulb but not whole brain tissue lysates (n = 6). 40 μg of protein were subjected to SDS-PAGE and immunoblotting. Representative immunoblots (left panel) and quantification (right panel) are shown. *, p<0.05 (student's t-test). (B) Dnmt1 is stabilized in RGS6−/− MEFs. WT and RGS6−/− MEFs were treated with CHX (100μM) for indicated time, lysed, and probed for Dnmt1 and Actin (loading control). Dnmt1 levels from triplicate experiments were quantified and expressed as % control levels. Two-way ANOVA revealed a significant effect of genotype (F1,24 = 130.55; p<0.0001) and time(F5,24 = 5.99; p=0.001). (C) Transfection of full length RGS6L but not RGS6LΔGGL (Fig. S5) suppressed Dnmt1 protein expression in RGS6−/− MEFs. (D) Co-immunoprecipitation of RGS6 and Tip60 in COS-7 cells co-transfected with RGS6-GFP and HA-Tip60. Cell lysates were subjected to immunoprecipitation with anti-RGS6 and probed for RGS6 and HA. (E) Co-immunoprecipitation of endogenous RGS6 and Tip60 in vivo. Whole brain lysates were subjected to immunoprecipitation with anti-Tip60 and probed for RGS6, Tip60 and Dnmt1. (F) The RGS domain of RGS6 was sufficient to mediate interaction between RGS6 and Tip60. GFP-RGS6 proteins were efficiently immunoprecipitated by anti-GFP from transfected COS-7 cell lysates despite some differences in their expression and precipitates were probed for GFP and Tip60. A schematic of truncation mutants used is depicted in Fig. S5. IgG heavy chains (HC) and light chains (LC) are labeled. (G) Loss of RGS6 impairs acetylation and ubiquitylation of Dnmt1. WT and RGS6−/− MEFs were transfected with pcDNA3-His-ubiquitin via Lipofectamine 2000 according to the manufacturer's protocol. Cell lysates were prepared and subjected to immunoprecipitation with anti-Dnmt1. Immunoprecipitates were probed for Dnmt1, His and acetylated-lysine. (H) Tip60 knockdown with shRNAs increases the Dnmt1 protein level in WT MEFs but not in RGS6−/− MEFs. 293T cells were co-transfected with envelope plasmid pVSV-G, packaging plasmid PAX2, and empty viral vector or viral DNA containing shTip60-1 or shTip60-2 (Open Biosystems) by electroporation as described(44). Twenty four hours after transfection, virus-containing culture medium was collected. WT and RGS6−/− MEFs were incubated with virus-containing medium for 48 hours. Representative immunoblot (upper panel) and summary of multiple experiments illustrating effects of Tip60 knockdown on Dnmt1 protein levels in WT and RGS6−/− MEFs (lower panel) are shown. Two-way ANOVA revealed a significant effect of genotype (F1,12 = 23.05; p=0.0004) and Tip60 knockdown (F2,12 = 11.84; p=0.0014). (F) Hypothetical model depicting RGS6 scaffolding of Tip60 and Dnmt1 to facilitate Tip60 acetylation of Dnmt1 and subsequent Dnmt1 polyubiquitylation and degradation. Tissue lysates were prepared and quantified as described previously(45). Immunoblotting and transfections were performed as previously described(7). Antibodies used were: RGS6 (prepared in the lab(7), 1:5000); HA (Santa Cruz, 1:1000); Tip60 (Santa Cruz N17, 1:1000); Dnmt1 (Santa Cruz, 1:1000); GFP (Santa Cruz, 1:2000); His (Santa Cruz, 1:1000); Acetylated-lysine (Cell signaling, 1:5000); Actin (Sigma, 1:2000); Tubulin (Millipore, MA; 1:5000). **, p<0.01; ***, p<0.001 (bonferroni multiple comparisons). Unless otherwise indicated all experiments were performed in triplicate and representative results are shown. Data are presented as mean ± S.E.M.