Abstract
Background
Community-acquired bacterial pneumonia (CABP) is a leading cause of morbidity and mortality especially in hospitalized patients. In place of clinical end points traditionally used to evaluate antimicrobial efficacy for its treatment, Food and Drug Administration guidelines now require all registration trials to assess clinical response at day 4. The primary objective of this study was to assess health outcomes (length of stay [LOS] and hospital charges) between responders and nonresponders at this time point.
Methods
The Premier database was used to identify adult patients from 4 participating hospitals with a principal diagnosis of CABP (International Classification of Diseases, Ninth Revision, Clinical Modification, codes 481, 482.0, 483.8, 484.3, 484.5, 485, 486, or 487.0) hospitalized between July 1, 2010, and June 30, 2011. Only non–intensive care unit patients with hospital stays exceeding 2 days and receiving intravenous antibiotic agents within 24 hours of admission were included. After institutional review board approvals, a retrospective chart review extracted data for patient demographics, clinical efficacy variables at day 4, LOS, and total hospital charges. Data analysis included multivariable gamma regression models to control for patient demographics and clinical differences between responders and nonresponders.
Results
A total of 666 patients met study the criteria. Mean (SD) age was 70.7 (17.9) years, and 42.5% were males. Among these patients, 277 (41.6%) achieved clinical response by day 4 of initial antibiotic therapy. The unadjusted mean (SD) LOS was 6.3 (2.8) days for responders and 7.4 (5.6) days for nonresponders (P = 0.0009). Respective unadjusted total hospital charges were $22,827 (SD, $17,724) and $26,403 ($36,882) (P = 0.0031). Adjusted for demographics and clinical factors, nonresponders compared with responders had an increased LOS of 0.9 days (8.4 vs 7.5 days; P = 0.0008), resulting in associated charges of approximately $2500 ($34,139 vs $36,629; P = 0.0768).
Conclusions
In this real-world chart study, less than half of hospitalized patients with CABP achieved clinical response at day 4 of initial intravenous antibiotic therapy. The observed clinical response was associated with a significantly shorter hospital stay and trended toward lower total hospital charges. These findings corroborate the Food and Drug Administration guidance for assessing antimicrobial therapy at day 4 because responder is associated with improved health outcomes.
Key Words: community-acquired pneumonia, clinical response, length of stay, hospitalization charges
Community-acquired pneumonia (CAP) is the most common form of pneumonia and is associated with significant morbidity and mortality.1,2 Pneumonia is the second leading cause of hospitalization in the United States with more than 1.2 million hospitalizations in 2006.3 Approximately 1 million episodes of CAP occur annually in adults 65 years and older in the United States. Patients in this age group are prone to increased rates of hospitalization and mortality.4 Although most CAP cases are treated as outpatients, hospitalized patients account for the largest proportion of pneumonia-related mortality and health care expenditures. Overall, mortality ranges from 5.1% for patients hospitalized or treated in an ambulatory setting to 36.5% for patients treated in an intensive care unit (ICU).5 Pneumonia-related hospitalization is associated with 4.6 deaths per 100 discharges, which is higher than the risk of mortality for heart disease (3.7/100) or for hospitalizations as a whole (4.2/100).6 The observed decrease in CAP-related mortality associated with the introduction of antibiotics has remained unchanged, whereas pneumonia-related rates of hospitalization have increased.7
Bacteria are the most common identifiable cause of CAP. Common etiologic agents include Streptococcus pneumoniae, Haemophilus influenzae, and Staphylococcus aureus. Streptococcus pneumoniae is the most common etiologic bacterium and has the highest associated mortality.8–10 Certain respiratory viruses and atypical bacterial pathogens such as Chlamydophila pneumoniae and Legionella pneumophila also cause CAP.11 Only half of the cases of CAP have an etiology microorganism identified.2 In cases where a microorganism is never identified, diagnosis is often based on history and physical examination and antibiotic treatment is usually empiric.
The Food and Drug Administration (FDA) has convened meetings and published guidance documents addressing both the need to better identify patients with community-acquired bacterial pneumonia (CABP) and to target drug development with early clinical response times at day 4, approximately 72 hours into the course of antimicrobial therapy.11 The FDA defines CABP as an acute infection of the pulmonary parenchyma associated with symptoms such as fever or hypothermia, chills, rigors, cough, chest pain, or dyspnea, accompanied by the presence of a new lobar or multilobar infiltrate on a chest radiograph. Based on these criteria, few studies in the literature assess clinical response rates and health outcomes (hospital length of stay [LOS] and total hospital charges) after 4 days of initial intravenous (IV) antibiotic therapy among hospitalized adult patients with CABP who have not been treated in the ICU. The purposes of this study were (1) to assess the clinical and patient characteristics of non-ICU hospitalized adult patients with CABP who did and did not achieve clinical responses on or before day 4 of initial IV antibiotic therapy and (2) to describe the impact on hospital LOS, total charges, and 30-day all-cause readmission in these 2 patient cohorts.
METHODS
Study Design and Patient Selection
This retrospective observational study included non-ICU hospitalized patients with CABP aged 18 years and older between July 1, 2010, and June 30, 2011, who had a primary diagnosis code of CABP and received IV antibiotic treatment within 24 hours of their admission. The primary diagnosis of CABP was identified with International Classification of Diseases, Ninth Revision, Clinical Modification (ICD-9-CM) codes of 481, 482.0, 483.8, 484.3, 484.5, 485, 486, or 487.0. Additional selection criteria included being admitted from home with a hospitalization of at least 4 days. Patients were excluded for probable health care–associated pneumonia, receiving dialysis, cancer chemotherapy, or wound care during their hospital stay, or prior hospitalization in the 30 days preceding the identified admission. The initial visit identified was used to analyze outcomes, and the first visit after the initial identified visit was used for readmission analysis. Patient-level data were collected through July 30, 2011, to evaluate 30-day all-cause readmission.
Data Source and Collection
This study used the Premier research database and medical chart review.12 The database is a large US hospital-based, service-level, all-payer, comparative database that contains information on approximately 5.5 million annual hospital discharges that represents approximately one fifth of all annual US hospitalizations primarily from nonprofit, nongovernmental, community, and teaching hospitals, and health systems. The Premier database includes hospitalizations from more than 500 hospitals for the period 2000 to the present. It consists of data from standard hospital discharge files that is extracted from deidentified patient daily service records. This includes patient demographics and disease states as well as information on billed services for medications, laboratory, diagnostics, and therapeutic services. Information on hospital characteristics such as geographic location, number of beds, and teaching status is also available. Detailed clinical information was extracted directly from medical chart reviews conducted at the 4 participating hospitals that submit discharge data to the Premier database to determine whether a patient with CABP achieved a clinical response on or before day 4 after initiation of antibiotic therapy. Regional and hospital institutional review boards provided study protocol approvals.
Patient Demographics, Comorbidities, and Risk Assessment
Patient demographics including age, sex, and race/ethnicity were examined. In addition, admission source and the comorbidities of type 1 or 2 diabetes mellitus (ICD-9 codes 250.00–250.93) and other underlying pulmonary disease (ICD-9 codes 470–478.99, 490–496.99, or 510–519.99) were identified. Indicators for alcohol use, smoking, and obesity were also recorded. Clinical data abstracted from the medical records from admission day 1 through day 4 included vital signs, clinical signs, details on the specific pathogen of infection, and antibiotic treatment, and data necessary to calculate risk assessment.
A mortality risk score was prospectively calculated by the researchers using confusion, uremia, respiratory rate, blood pressure, and age 65 years and older (CURB-65) data extracted from the medical records.13 A score of 0 to 1 indicates that the patient should be treated as an outpatient, a score of 2 signals the clinician to consider a short hospital stay or watch very closely as an outpatient, and a score of 3 to 5 requires hospitalization with consideration as to whether the patient needs to be in the ICU. Morbidity before discharge was determined using the 3M All Patient Refined Diagnosis Related Group (APR-DRG) severity of illness (SOI),14 which accounts for age and the clinical severity of primary diagnosis and all secondary diagnoses assigned in the course of hospitalization. This severity classification was computed by the established 3M algorithm at the time of hospital discharge using the Premier database.
Treatment Response and Health Outcomes
Response to antibiotic therapy was determined using the FDA-defined clinical stability and symptom resolution criteria. Patients were classified as stable if the following conditions were met by day 4: temperature of less than or equal to 37.8°C (100.4°F), heart rate of less than or equal to 100 beats per minute, respiratory rate of less than or equal to 24 breaths per minute, systolic blood pressure of more than or equal to 90 mm Hg, oxygen saturation of more than or equal to 90%, absence of confusion/disorientation, and no worsening of baseline symptoms with improvement of at least 2 symptoms (ie, cough, dyspnea, chest pain, or sputum production). Pulse rate was used as a surrogate marker for heart rate. By these criteria, patients deemed as responders were compared with those patients who did not respond by day 4.
Length of hospital stay in days was measured during the hospitalization. Total visit charges were defined as the dollar amounts billed by the hospital to the payer; however, the amount reimbursed to the hospital was not available. Readmission was defined as a return by the patient to the same hospital within 30 days of discharge for any cause.
Statistical Analyses
Descriptive analysis was used to describe the populations and patient characteristics. Continuous data were expressed as mean, SD, and median; categorical data were expressed as counts and percentages. Bivariate analysis tables display overall results from the CABP population and statistical comparisons between day 4 responders and nonresponders for study variables. For continuous variables, Wilcoxon rank sum tests were used to assess statistical significance of the differences. For categorical variables, χ2 tests were used to assess statistical significance of the differences. All statistical tests were performed using a 2-sided test with α of 0.05. Multivariable gamma regression was used to model estimates for the mean of hospital LOS and total hospital charges adjusting for demographic and clinical variables. Backward elimination was used to determine the covariates that were used in the final models. A logistic model was also constructed to assess the association between day 4 response and 30-day readmission, controlling for the same covariates in the gamma regression model. All statistical analyses were performed using SAS (SAS v9.2; SAS Institute Inc, Cary, NC).
RESULTS
Patient Demographics and Risk Assessment
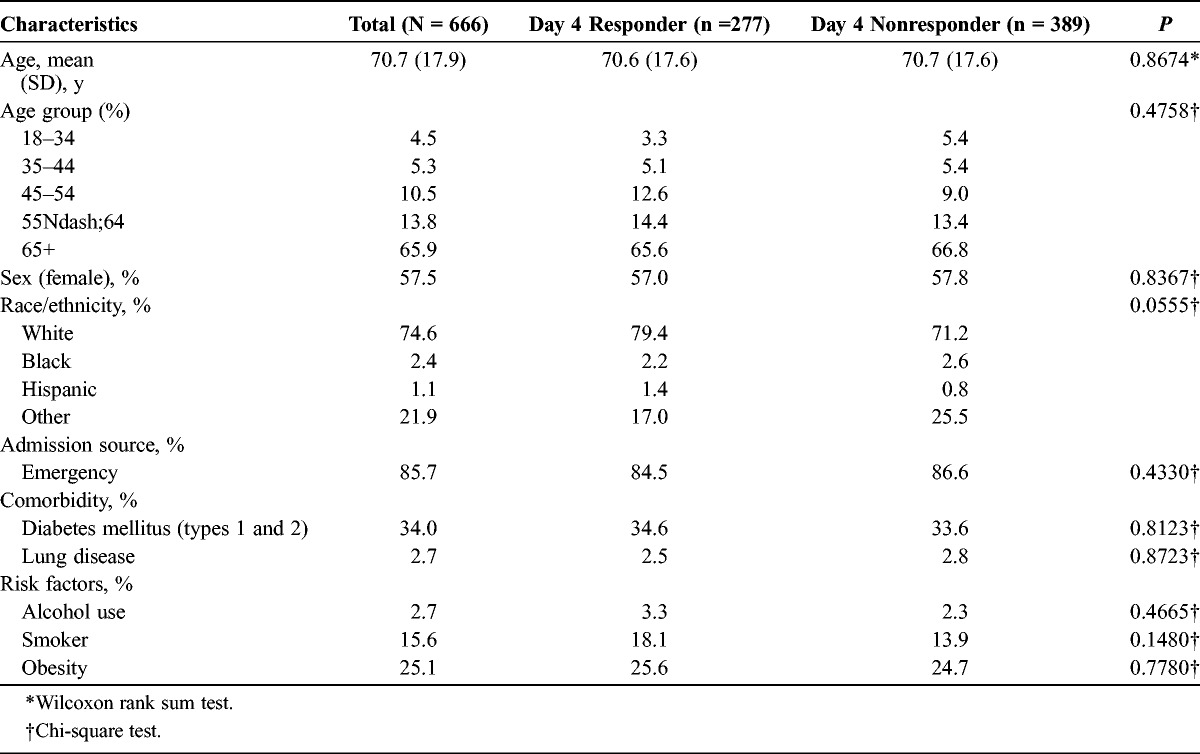
There were 666 patients who met the selection criteria (Table 1). The mean age (SD) for the overall study population was 70.7 (17.9) years. Most patients were female (57.5%) and white (74.6%). The presence of comorbidities for type 1 and 2 diabetes mellitus and underlying lung disease was 34.7% and 65.3%, respectively. The number of patients with CABP with alcohol use, smoking, and obesity was 18 (2.7%), 104 (15.6%), and 164 (24.6%), respectively. There were 277 (41.6%) patients who reached responder status by day 4 and 389 (58.4%) patients who were classified as day 4 nonresponders. Patients from these respective cohorts were similar in age (70.6 [SD, 17.6] years vs 70.7 [17.6] years), sex (female, 57.0% vs 57.8%), and race (white, 79.4% vs 71.2%). Comorbidities and risk factor indicators also were comparable and not statistically significant.
TABLE 1.
Patient Demographics, Admission Source, Comorbidities, and Risk Factor Indicators
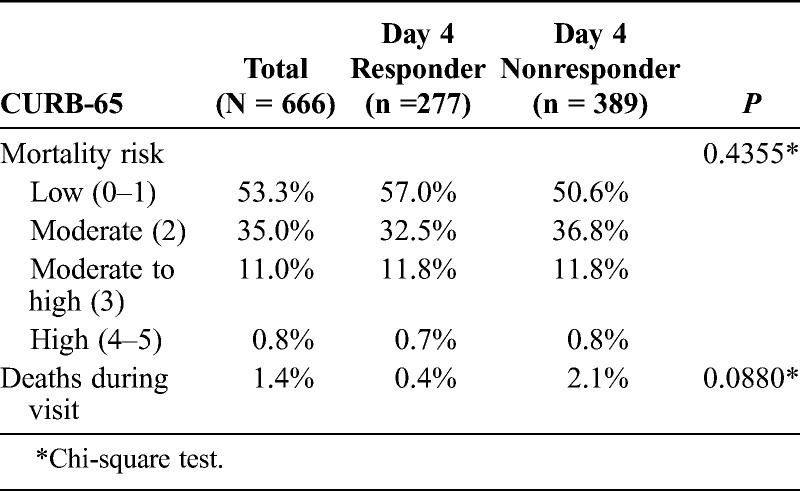
The overwhelming majority of patients with CABP (88.3%) had a low (53.3%) to moderate (35.0%) mortality risk as assessed by CURB-65 scores of 0 to 1 and 2, respectively (Table 2). The remainder of patients fell into the moderate to high (11.0%; score of 3) and high (0.8%; score of 4–5) risk categories. Nine (1.4%) patients died during the hospitalization, 1 (0.4%) patient from the responder group and 8 (2.1%) patients from the nonresponder group.
TABLE 2.
The CURB-65 Score
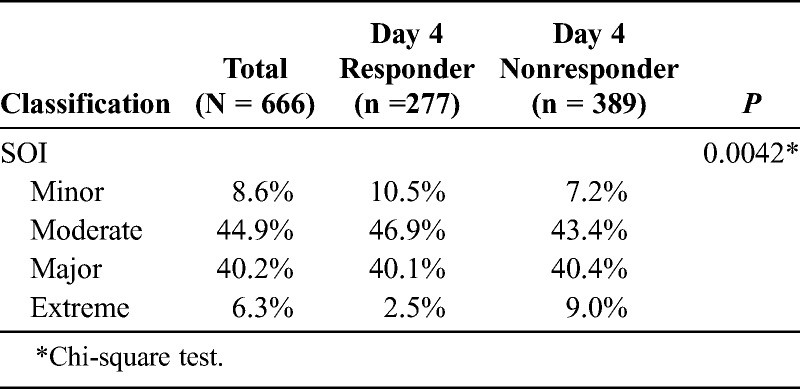
The 3M APR-DRG SOI classification is represented in Table 3. Most of the patients with CABP were classified as moderate (44. 9%) and major (40.2%). More than 80% of the day 4 responders and nonresponders were also categorized with either moderate or major SOI. The statistical significance (P = 0.0042) observed between these groups is mainly seen in the tails of distribution for minor and extreme SOI. More nonresponders were classified as extreme. A score of 0 to 1 indicates that the patient should be treated as an outpatient, a score of 2 signals the clinician to consider a short hospital stay or watch very closely as an outpatient, and a score of 3 to 5 requires hospitalization with consideration as to whether the patient needs to be in the ICU.
TABLE 3.
The 3M APR-DRG SOI Classification
Treatment Response and Health Outcomes
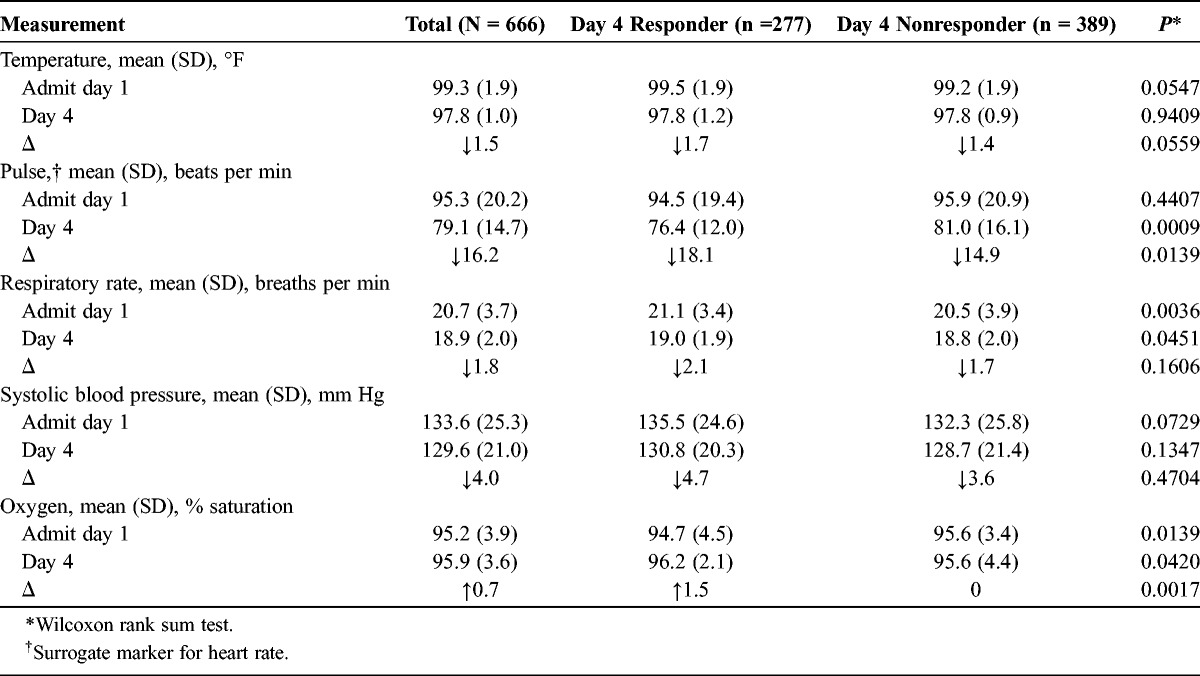
Clinical parameters and symptoms are displayed in Table 4. In the overall patient with CABP group, higher body temperatures, pulse and respiratory rates, and systolic blood pressures were seen at admission when compared with day 4. Oxygen saturation was within normal limits at day 1 admission and remained essentially unchanged at day 4. At the time of admission, the mean body temperature was slightly higher in the responder group than the day 4 nonresponders (99.5°F vs 99.2°F; P = 0.0547). After 4 days of antibiotic therapy, the body temperatures decreased by 1.7°F and 1.4°F (P = 0.0559), respectively, to 97.8°F in both groups. Admission pulse rates were similar between patient groups and decreased significantly in the responder group when compared with the nonresponder group after antibiotic therapy. The respective differences in pulse rates were significant (18.1 beats per minute vs 14.9 beats per minute; P = 0.0139). Respiratory rates were higher in the responders versus the nonresponders at both time of admission and day 4 of therapy. The decrease in respiratory rate between the periods was similar between the 2 patient populations (2.1 breaths per minute vs 1.7 breaths per minute; P = 0.1606). Admission and day 4 systolic blood pressures were similar between the responders and nonresponders, and the observed reductions at 4 days of treatment were not significantly different. Oxygen saturation levels were normal at admission and throughout the hospitalization. It rose slightly from admission to day 4 in the responders and remained unchanged in the nonresponders.
TABLE 4.
Clinical Stability Parameters and Symptoms
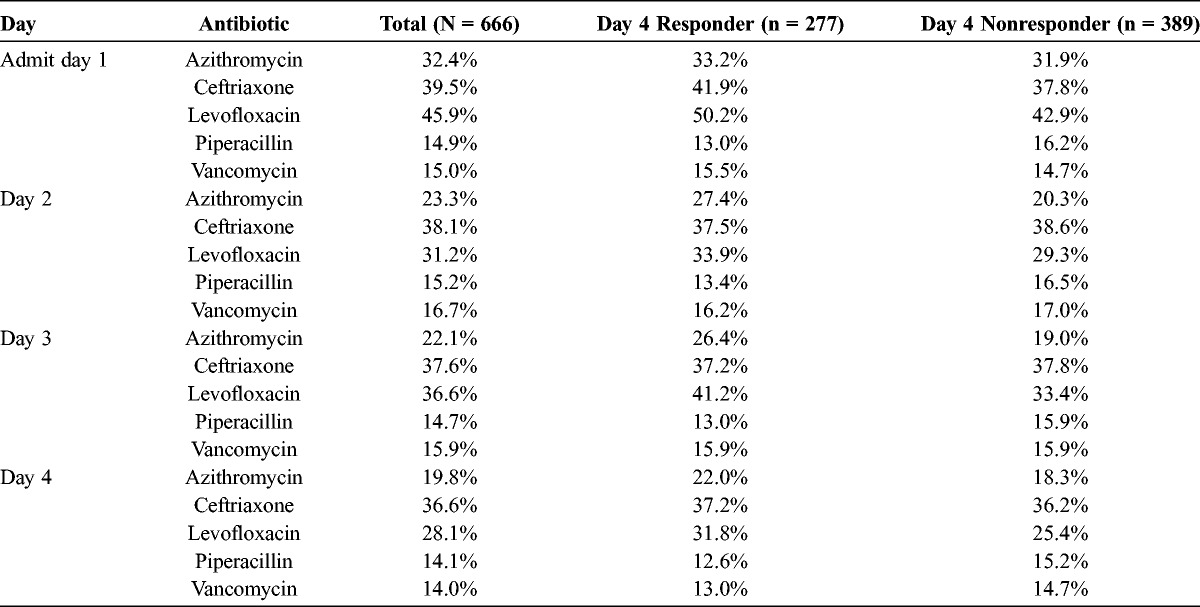
Approximately 97% of the study population had medical chart confirmation of IV antibiotic administration on day of admission. Most patients (>85%) continued the therapy through day 4. Table 5 lists the most frequently used antibiotics by day of treatment of responders and nonresponders. Ceftriaxone or levofloxacin were administered to the largest percentage of patients; approximately one third of patients received ceftriaxone, and one third of patients received levofloxacin.
TABLE 5.
Admission Through Day 4 of Most Frequently Prescribed Antibiotics
Health outcomes and 30-day all-cause readmissions are summarized in Table 6. The hospital LOS for the overall CABP population was 6.9 (SD, 4.6) days with total visit charges of $24,916 (SD, $30,449). The unadjusted mean hospital LOS was shorter in the responder group than in the day 4 nonresponder group (6.3 [SD, 2.8] days vs 7.4 [5.6] days; Δ = 1.1 days; P = 0.0009). The unadjusted means for total hospital charges incurred to payer were significantly lower in the responder group ($22,827 [SD, $17,724] vs $26,403 [$36,882]; Δ = $3576; P = 0.0031).
TABLE 6.
Unadjusted and Adjusted Outcomes for Hospital LOS, Charges, and 30-Day All-Cause Readmissions
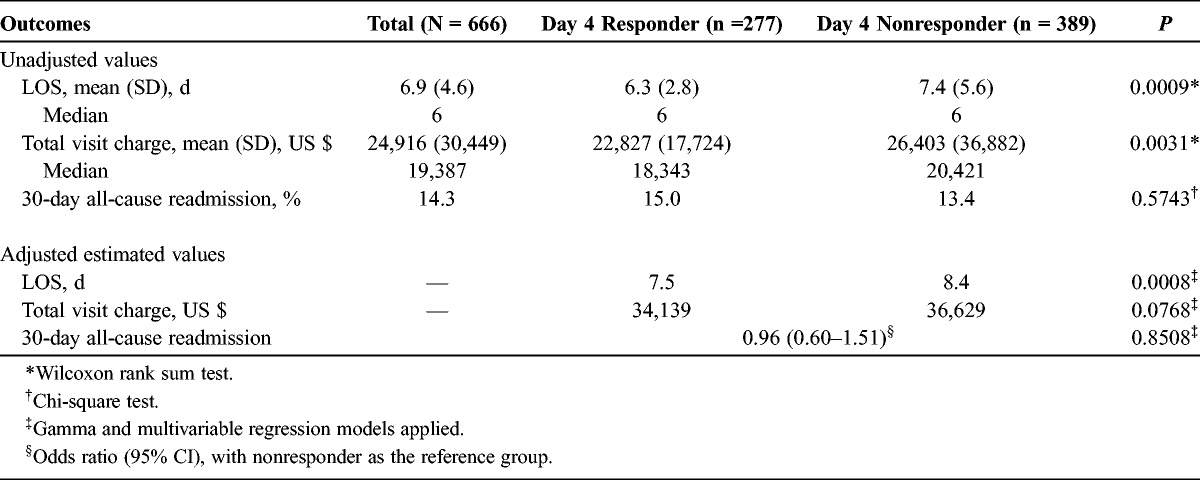
After adjusting for the significant covariates that included admission source, 3M APR-DRG SOI, alcohol use, and smoker status, the day 4 responders compared with the nonresponders had a significantly shorter mean LOS [7.5 (95% confidence interval [CI], 6.8–8.3) vs 8.4 (95% CI, 7.6–9.2); P = 0.0008). The respective average total charges to the payer showed a numerical difference ($34,139 [95% CI, $30,001−$38,844] vs $36,629 [95% CI, $32,225−$41,635]; P = 0.0768).
The unadjusted 30-day all-cause readmissions were 15.0% versus 13.4% (P = 0.5743) for day 4 responders and nonresponders, respectively. After adjusting for the significant covariates, the odds ratio for 30-day all-cause readmission was 0.96 (95% CI, 0.60–1.51; P = 0.8508) for the responder group versus the nonresponder group (reference group).
DISCUSSION AND CONCLUSIONS
Before the recent FDA recommendations, clinical response in patients with CAP was assessed during the first 7 days of antibiotic therapy, and the outcomes were categorized as early clinical deterioration or improvement, late clinical deterioration or improvement, and finally, lack of clinical improvement.2 The present study looked at the clinical improvement at day 4 and is the first retrospective chart study assessing day 4 response rates in a real-world clinical practice since the latest release of the FDA guidelines. In our study, patients identified as day 4 responders with clinical improvement after their course of IV antibiotics had shorter hospital LOS and lower total hospital charges. This is the first study of its kind to examine day 4 responder status in relation to hospital LOS and charges.
In the latest FDA briefing document on “Endpoints and Clinical Trial Issues in Community-Acquired Bacterial Pneumonia” (November 2011), day 4 of therapy (approximately 72 hours into the course of therapy) is indicative of response to therapy.11 To highlight the significance of these new guidelines, 2 phase 3 clinical trials (Ceftaroline Community Acquired Pneumonia Trial versus Ceftriaxone in Hospitalized Patients [FOCUS] 1 and FOCUS 2) that used clinical cure rates at the test-of-cure visit and previously demonstrated noninferiority of ceftaroline versus ceftriaxone in the treatment of non-ICU hospitalized patients with CABP was reevaluated at day 4. The investigators demonstrated that ceftaroline seemed to provide clinical benefit over ceftriaxone at day 4 and concluded that this end point might be more clinically relevant with respect to hospital discharge and oral step-down therapy.15 Using real-world data from US hospitals, our study was specifically designed to address the potential benefit to health outcomes and demonstrated shorter hospitalization and reduced hospital charges in patients who were responsive to treatment at or by day 4. To date, a limited number of publications exist on patient outcomes (LOS, total charges for care, and resource use) and clinical response at day 4 of initial IV antibiotic therapy in hospitalized adult non-ICU patients with CABP.16
In this study, the lack of statistical significance for total hospital charges may be explained by the relative small sample size given the large variance of charge data. It is therefore plausible that if the hospital-reported charges in this study had been more homogeneous, the $2490 difference between the mean hospital charges (adjusted) may have been statistically significant lower for responders than nonresponders. This is further supported by the difference in adjusted hospital LOS, which is a known driver of hospital charges (significantly lower for responders by almost 1 day).
The strengths of this study include the depth and breadth of the Premier database, which uses complete hospital census data rather than a statistical sample of patients. Chart review to capture clinical data allowed for assessment of day 4 clinical improvement, which meant that direct clinical data added to the strength of the administrative information. From the payer perspective, charges are easily understood in relation to the expected payment rates. Most of the payers that base reimbursement on charges pay negotiated or discounted amounts; other insurers and self-pay patients are still understanding of charges in relation to payment, so this is a well-understood measure of the economic aspects of care that supports the value of the study results.
This study has several limitations that are primarily associated with the use of administrative databases, including reliance on accurate and complete ICD-9 coding, as well as potential issues with the accuracy of billing (charge master data) to identify imaging and other diagnostic tests used in the care of these patients. In addition, documented pathogens could only be identified in 4.7% of the study population, hampering our ability to conduct an analysis on appropriateness of treatment, a potential confounder of the analysis. Lastly, database and medical chart information from only 4 selected high-volume hospitals could limit the generalizability of the study findings to other hospital settings.
In conclusion, this is the first study to assess the association between clinical response at day 4 of IV antibiotic treatment and health outcomes in hospitalized patients with CABP using real-world data. The results from the study show that clinical response at day 4 is economically meaningful, associated with shorter hospital stays and lower total hospital charges. These findings, from a health outcomes perspective, support the FDA guidance for assessing antimicrobial therapy at day 4.
ACKNOWLEDGMENT
The authors thank Bernadette H. Johnson for her help in data analysis and Carol Cohen who provided editorial feedback for the article.
Footnotes
This work was funded by Forest Research Institute, a subsidiary of Forest laboratories, Inc.
This study was supported by Forest Laboratories, Inc. Forest Laboratories, Inc was involved in the design and decision to present these results. Forest Laboratories, Inc had no involvement in the collection of data but was involved in the analysis and interpretation of the results. Scott Robinson, Frank Ernst, and Craig Lipkin are employees of Premier Research Services, who received funding from Forest Research Institute to conduct this analysis. Xingyue Huang is an employee of Forest Research Institute.
This work was presented in part at the 53rd Interscience Conference on Antimicrobial Agents and Chemotherapy 2013 conference in Denver, Colo.
REFERENCES
- 1. Restrepo MI, Anzueto A. The role of new therapies for severe community-acquired pneumonia. Curr Opin Infect Dis. 2006; 19: 557– 564. [DOI] [PubMed] [Google Scholar]
- 2. Mandell LA, Wunderink RG, Anzueto A, et al. Infectious Diseases Society of America/American Thoracic Society consensus guidelines on the management of community-acquired pneumonia in adults. Clin Infect Dis. 2007; 44 (suppl 2): S27– S72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Agency for Healthcare Research and Quality. Pneumonia most common reason for hospitalization. Available at: http://archive.ahrq.gov/news/nn/nn070208.htm. Accessed September 2013.
- 4. Fry AM, Shay DK, Holman RC, et al. Trends in hospitalizations for pneumonia among persons aged 65 years or older in the United States, 1988-2002. JAMA. 2005; 294 (21): 2712– 2729. [DOI] [PubMed] [Google Scholar]
- 5. Fine MJ, Smith MA, Carson CA, et al. Prognosis and outcomes of patients with community-acquired pneumonia. A meta-analysis. JAMA. 1996; 275 (2): 134– 141.8531309 [Google Scholar]
- 6. DeFrances CJ, Hall MJ. 2005 National hospital discharge survey. Adv Data. 2007; (385): 1– 19. [PubMed] [Google Scholar]
- 7. Gilbert K, Fine MJ. Assessing prognosis and predicting patient outcomes in community-acquired pneumonia. Semin Respir Infect. 1994; 9 (3): 140– 152. [PubMed] [Google Scholar]
- 8. Arnold FW, Summersgill JT, Lajoie AS, et al. A worldwide perspective of atypical pathogens in community-acquired pneumonia. Am J Respir Crit Care Med. 2007; 175 (10): 1086– 1093. [DOI] [PubMed] [Google Scholar]
- 9. Howard LS, Sillis M, Pasteur MC, et al. Microbiological profile of community-acquired pneumonia in adults over the last 20 years. J Infect. 2005; 50 (2): 107– 113. [DOI] [PubMed] [Google Scholar]
- 10. Weiss K, Low DE, Cortes L, et al. Clinical characteristics at initial presentation and impact of dual therapy on the outcome of bacteremic Streptococcus pneumoniae pneumonia in adults. Can Respir J. 2004; 11 (8): 589– 593. [DOI] [PubMed] [Google Scholar]
- 11.Federal Drug Administration. Endpoints and Clinical Trial Issues in Community-Acquired Bacterial Pneumonia. Anti-Infective Drugs Advisory Committee. 2011. Available at: http://www.fda.gov/downloads/advisorycommittees/committeesmeetingmaterials/drugs/anti-infectivedrugsadvisorycommittee/ucm275823.pdf. Accessed September 2013.
- 12.Premier Research Services Database Information [Premier, Inc Web site]. Available at: http://www.premierinc.com/prs/. Accessed September 2013.
- 13. Lim WS, van der Eerden MM, Laing R, et al. Defining community acquired pneumonia severity on presentation to hospital: an international derivation and validation study. Thorax. 2003; 58 (5): 377– 382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Averill RF, Goldfield N, Hughes JS, et al. 3M APR DRG Classification System Methodology Overview. Clinical Research and Documentation Departments of 3M Health Information Systems; Wallingford, CT and Murray, UT; 2008. [Google Scholar]
- 15. Eckburg PB, Friedland DH, Llorens LL, et al. Day 4 clinical response of ceftaroline fosamil versus ceftriaxone for community-acquired bacterial pneumonia. Infect Dis Clin Pract. 2012; 20 (4): 254– 260. [Google Scholar]
- 16. Halm EA, Fine MJ, Marrie TJ, et al. Time to clinical stability in patients hospitalized with community-acquired pneumonia: implications for practice guidelines. JAMA. 1998; 279 (18): 1452– 1457. [DOI] [PubMed] [Google Scholar]


