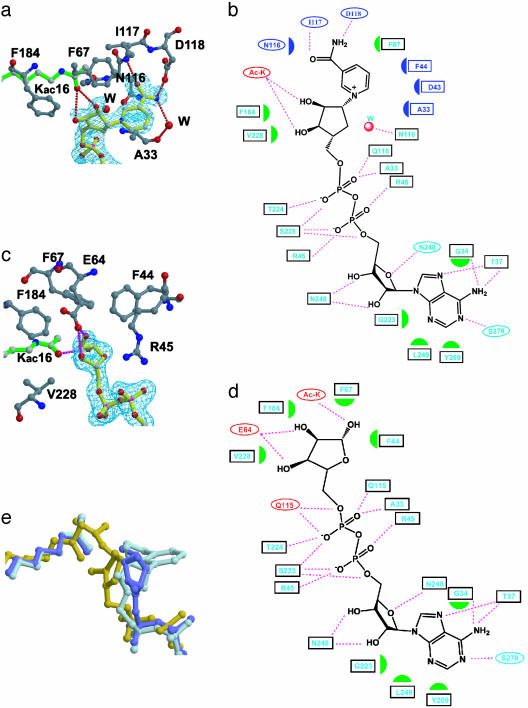
Fig. 2.
yHst2-NAD analog interaction. (a) The carba-NAD+-binding site of yHst2 highlighting the yHst2 residues (carbon, gray; oxygen, red; and nitrogen, blue) that contact the nicotinamide and nicotinamide-ribose (carbon, yellow). A simulated annealing Fo – Fc omit map of carba-NAD+ is counted at 3.0 σ (cyan) and the acetyllysine substrate (green) is also highlighted. (b) Summary of yHst2 carba-NAD+ interactions. Hydrogen bonds are indicated with dashed lines and van der Waals interactions are indicated with half-moon symbols. The residues highlighted in cyan take part in conserved interactions with the yHst2/2′-O-acetyl-ADP-ribose and yHst2/ADP-ribose complexes. The residues colored in red take part in nonconserved interactions and the residues highlighted in blue take part in interactions with the nicotinamide group. (c) The ADP-ribose-binding site of yHst2, highlighting the yHst2 residues that contact the nicotinamide and nicotinamide-ribose. A simulated annealing Fo – Fc omit map of ADP-ribose counted at 3.0 σ and the color coding are as described for a. (d) Summary of yHst2–ADP-ribose interactions. The coding for interactions and color designations are as described for b. (e) Overlay of the carba-NAD+ (light blue), ADP-ribose (dark blue), and 2′-O-acetyl-ADP-ribose (yellow) ligands, using the corresponding proteins for the superpositions. The acetyl group from the acetyllysine substrate of the respective complex is also shown in the same color.