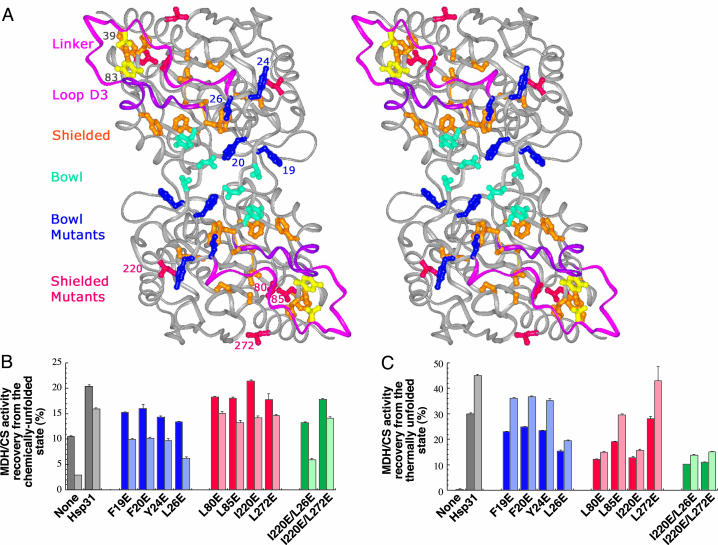
Fig. 1.
Effect of glutamate substitutions in bowl and linker-loop-shielded residues on Hsp31 chaperone activity. (A) Stereoview of Hsp31 with bowl residues shown in cyan and blue (mutated residues) and linker-loop-shielded residues shown in orange and red (mutated residues). The linker (pink), loop (purple), and residues converted to cysteines (yellow) are shown also. (B) Gdn·HCl-unfolded MDH (dark bars) or urea-unfolded CS (light bars) was diluted in buffer containing Hsp31 variants. Samples were assayed after 3 h (MDH) or 30 min (CS) of incubation at 23°C. (C) MDH (dark bars) or CS (light bars) were mixed with Hsp31 variants, incubated for 30 min at 45°C, and assayed after 30 min at 23°C. Enzymatic activities measured with Hsp31 bowl mutants are shown in two shades of blue. Those measured in the presence of Hsp31 variants mutated in linker-loop-shielded residues are shown in two shades of red. Activity results for double mutants are shown in two shades of green. Error bars were obtained for three independent replicates of activity measurements that were each performed in triplicate.