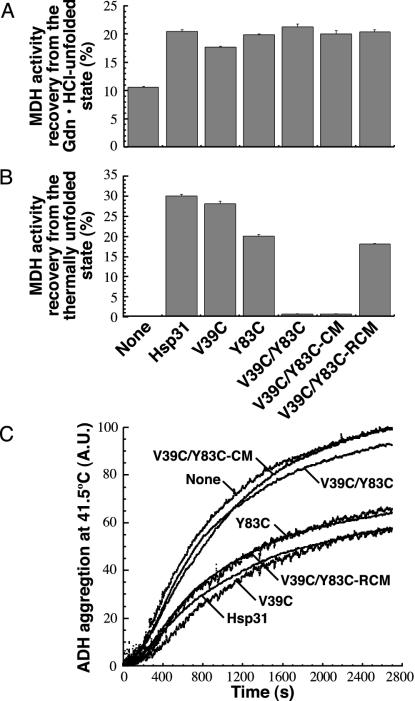
Fig. 4.
Linker mobility is essential for Hsp31 substrate binding at high temperatures. (A) Gdn·HCl-unfolded MDH was diluted into buffer containing Hsp31, the single cysteine mutants, or the three forms of the double cysteine mutant. Samples were assayed after 3 h at 23°C. (B) MDH was mixed with Hsp31 variants, incubated 30 min at 45°C, and assayed after 30 min at 23°C. Error bars were obtained for three independent replicates of activity measurements that were each performed in triplicate. (C) ADH thermal aggregation was monitored by light scattering in the presence of the indicated Hsp31 variants. Results are reported in arbitrary units (A.U.).