Abstract
Lack of adherence to continuous positive airway pressure therapy (CPAP) limits the effectiveness of treatment of obstructive sleep apnea (OSA). We hypothesized that an irregular bedtime would be negatively related to regular use of CPAP treatment. If so, modifying bedtime schedule may address the persistent problem of inconsistent CPAP use in adults with OSA. In a prospective longitudinal study, we examined whether inconsistent self-reported bedtime before initiation of CPAP treatment, operationalized as bedtime variability, was (1) different among those adherent (≥ 4 hours per night) and non-adherent to CPAP treatment at one week and one month; and/or (2) was related to one-week and one-month CPAP use when other variables were accounted for. Consecutively-recruited newly-diagnosed OSA adults (N=79) completed sleep diaries prior to CPAP treatment. One-week and one-month objective CPAP use data were collected. Pre-treatment bedtime variability was different among CPAP non-adherers and adherers at one month and was a significant predictor of non-adherence at one month in multivariate analyses. The odds of one-month CPAP non-adherence were 3.5 times greater in those whose pre-treatment bedtimes varied by >75 minutes. Addressing sleep schedule prior to CPAP initiation may be an opportunity to improve CPAP adherence.
Keywords: Obstructive Sleep Apnea, Continuous Positive Airway Pressure, Patient Compliance, Sleep Diary
Non-adherence to continuous positive airway pressure (CPAP) is a well-recognized limitation to the effective treatment of obstructive sleep apnea (OSA) (Crawford, Espie, Bartlett, & Grunstein, 2014; Sawyer et al., 2011; Weaver & Grunstein, 2008). Patterns of CPAP use are established early in the treatment period, notably during the first week of home therapy; these early patterns of CPAP use are consistent with long-term CPAP use outcomes (Aloia, Arnedt, Stanchina, & Millman, 2007; Budhiraja et al., 2007; Weaver et al., 1997). It is therefore imperative to determine predictors of CPAP non-adherence prior to, or at, treatment initiation, in order to prevent or minimize early CPAP non-adherence.
To date, pre-treatment factors that have been examined include demographic/patient characteristics; titration method; sleep quality during titration polysomnogram; disease characteristics such as severity, symptom presentation, and nasal resistance; psychological factors such as outcome expectancies and self-efficacy; and social influences such as spousal/partner relationship types and partner sleep quality (Crawford et al., 2014). Although some important insights into CPAP non-adherence have emerged from this line of inquiry, the field continues to be challenged to develop effective intervention strategies that address CPAP non-adherence outcomes (Quan, Awad, Budhiraja, & Parthasarathy, 2012; Sawyer et al., 2011; Weaver, 2013).
In medication adherence studies, a consistent schedule of pill-taking, cueing for the behavior, and self-awareness of behaviors that support pill-taking habits are important in compliance, or adherence (McDonald, Garg, & Haynes, 2002; Schlenk, Burke, & Rand, 2001). Although CPAP treatment is certainly a different, and possibly more complex, health behavior than most medication regimens, “schedule” or “routine” has not been previously examined as a potentially influential pre-treatment factor on CPAP non-adherence. In our mixed-methods research on differences between CPAP adherers and non-adherers, non-adherent CPAP patients struggled with the “getting used to it” period, during which non-adherers identified incorporating CPAP into their daily routine as a treatment use barrier (Sawyer, Deatrick, Kuna, & Weaver, 2010). When daily routine is conceptualized as a possible modifiable factor of importance for the everyday application and use of CPAP, nightly bedtime routines may be an important influence on CPAP non-adherence. We therefore hypothesized that inconsistency of bedtime, operationalized as bedtime variability, would be related to CPAP non-adherence.
In the larger prospective, longitudinal study, the primary research objective was to develop and test a CPAP non-adherence risk assessment questionnaire (Sawyer et al., 2014). The current report addresses our secondary objectives: (1) To determine if pre-treatment bedtime variability among CPAP-treated adults with OSA, defined as the variation in time of going to bed across a defined period of one week, was different among CPAP adherers and non-adherers at one week and one month after CPAP initiation; and (2) To determine if pre-treatment bedtime variability was related to CPAP use at one week and one month. We also explored whether gender or socioeconomic status moderated these relationships, as recent evidence has suggested these characteristics are linked to CPAP non-adherence (Billings et al., 2011; Guralnick, Pant, Minhaj, Sweitzer, & Mokhlesi, 2012; Ye et al., 2012).
Methods
A prospective, longitudinal study was conducted at two clinical sleep centers in the US. One site was a large, academic-medical center-affiliated sleep center in a suburban region, and the second site was a community-based sleep center in a suburban setting. The study was approved by the institutional review boards of the principal investigator’s institution and the respective study sites. Written informed consent, which included HIPAA authorization, was obtained from all study participants by an investigator.
Participants
Participants were consecutively recruited from those referred to the sleep centers for CPAP titration polysomnogram (PSG). An open letter of invitation to participate in the study was distributed. Patients who provided their contact information to the study team in response to the invitation letter were contacted by a study investigator for pre-enrollment screening. Screening criteria included: (1) willingness to schedule a first research visit for informed consent discussion; (2) diagnostic PSG with a diagnosis of OSA based on the inclusion criteria for apnea-hypopnea index (AHI) ≥ 5 events/hr; (3) willingness to attend a CPAP titration PSG; and (4) no previous exposure to CPAP treatment. Patients with positive pre-enrollment screening were invited to participate in the study.
Inclusion criteria for the study were: (1) newly-diagnosed OSA with AHI ≥ 5 events/hour on in-laboratory PSG, conducted and scored in accordance with standard criteria (Iber, Ancoli-Israel, Chesson, & Quan, 2007); (2) referral to CPAP titration PSG; (3) able to speak and read English. Exclusion criteria were: (1) supplemental oxygen or bilevel positive airway pressure required during titration PSG; (2) new diagnosis of psychiatric disorder within six months prior to study enrollment; and (3) any medical contraindication to using CPAP for treatment of OSA (e.g., emergence of complex sleep apnea with exposure to CPAP). All potential participants were CPAP-naïve, with no previous exposure or experience with CPAP prior to study enrollment.
A total of 102 patients originally were enrolled. Two participants were lost to follow-up, two refused CPAP during titration PSG, and one did not return for titration PSG, resulting in 97 participants with complete primary outcome data (CPAP use). Complete sleep diary data was available for 79 participants. Participants with incomplete sleep diary data not included in the present analysis (n=18) did not differ on any baseline characteristic from those participants with complete sleep diary data.
An a priori power analysis was conducted to address the primary research objective (Sawyer et al., 2014). For the current analysis, which addressed secondary research objectives, the evaluation of confidence intervals is the preferred method for drawing conclusions about the size and direction of the effect, which are dependent on the variability of the data and the study sample size (Hoenig & Heisey, 2001; Walters, 2009). For this reason, confidence intervals for odds ratios/adjusted odds ratios were examined in non-significant results.
Measures
Demographic questionnaire
An 18-item questionnaire eliciting self-reported demographic information was employed at study enrollment.
Diagnostic PSG
Studies were scored based on standard criteria (Iber et al., 2007) using the alternative hypopnea definition, a 50% decrement in nasal pressure of 10 seconds or more, and ≥ 3% desaturation (Iber et al., 2007). Variables extracted from the diagnostic PSG included AHI and oxygen nadir in non-rapid eye movement (NR). Weight and height for body mass index (BMI) were extracted from the diagnostic PSG report. AHI, NR oxygen nadir, and BMI descriptive variables were included for sample description.
Sleep diary
A one-week sleep diary was completed by participants after diagnostic PSG but prior to CPAP titration PSG. Although other recording intervals of sleep diary data were considered (i.e., two weeks, one month), a one-week record was determined to be feasible within the protocol period of the study (i.e., between diagnostic and CPAP titration PSG). The investigators also considered the one-week diary record to be a reasonable burden for participants without risk of large amounts of missing data.
Participants were instructed to complete the diary for a consecutive seven-day period. Participants were asked to record the date, day of week, whether the day was a work day, vacation day, and/or weekend day, time to bed, and time of terminal awakening. Sleep diary instructions directed participants to enter their evening diary data in the 30 minutes preceding going to bed and their morning diary data within 30 minutes of arising from bed. The independent variable, pre-treatment bedtime variability, was derived from sleep diary recordings of self-reported time to bed.
Sleep diary data was considered complete if ≥ 5 consecutive days of data was recorded, so at least 70% of one-week diary data were available for analysis. Sleep diary data were complete for 79 participants. Four diary records had fewer than seven consecutive days recorded but met the inclusion criteria and were included in the analysis. Three of these included two consecutive weekend days (Friday-Saturday or Saturday-Sunday), and one included only weekdays.
CPAP use
Objective CPAP use data were collected from the internal microprocessor on standard CPAP devices after one month of home CPAP treatment. To reduce participant burden in comparison to longer follow-up, one-week and one-month CPAP use were the primary outcomes, based on extant evidence (Aloia et al., 2007; Budhiraja et al., 2007; Weaver et al., 1997) that early CPAP use (i.e., one week) is predictive of later CPAP use (i.e., one month, three months, six months, twelve months).
The majority of standard CPAP devices are equipped with an internal microprocessor that records all CPAP use periods. The devices used in this study, all Respironics™ devices, had internal sensors for pressure and flow detection. Based on a device-specific industry-developed algorithm for pressure and airflow detection, the device records the duration that CPAP was powered on and the duration of CPAP at effective pressure >20 minutes.
The CPAP use data were hours per night at effective pressure > 20 minutes. Non-adherence was defined as < 4hours/night of CPAP use. Clinical providers use this benchmark of CPAP non-adherence, as do researchers and the majority of third-party payers, guided by the Centers for Medicare & Medicaid Services policy for reimbursement of home CPAP therapy (2008). Although the evidence behind this commonly-applied cut-point has not been well-defined, in light of third-party payer policies to reimburse CPAP treatment only when CPAP use is over four hours/night for a 30-day period of treatment, studies of CPAP adherence/non-adherence are likely to persist with this dichotomized outcome. CPAP use was therefore dichotomized at <4 hours/night and ≥4 hours/night for logistic regression analysis. An average of less than 4 hours/night of CPAP use was defined as non-adherence; ≥ 4 hours/night average CPAP use was defined as adherence.
Covariates
Based on a review of literature (Sawyer et al., 2011), covariates were included that have been consistently linked to CPAP non-adherence: gender, race, marital status, education, and OSA severity (AHI, oxygenation). Other covariates, such as shiftwork, employment, and referral source, were selected for exploration based on their hypothesized relationship to the outcome of interest. Categorical covariates were gender, race (White/non-White), ethnicity (Hispanic/non-Hispanic), marital status (married/not married), education (any college/no college), employment (full-time/all other), rotating shift (yes/no), night shift (yes/no), referral source (primary care/all other), and AHI group (mild/moderate/severe). Continuous covariates were BMI, AHI, NR oxyhemoglobin nadir, and time < 90% oxyhemoglobin saturation (minutes).
Protocol
After informed consent, participants completed a demographic questionnaire. Medical history, medications, and diagnostic PSG variables were extracted from the electronic medical record. A paper copy of a pre-formatted sleep diary was provided to participants. Standardized instructions for sleep diary completion using an instruction template were verbally provided to participants by an investigator who was responsible for data collection at both sites. The sleep diary form included the same instructions printed at the top of the sleep diary for participant reference when completing the diary at home. Participants were instructed to complete the diary prior to returning to the sleep center for the CPAP titration PSG. Participants returned the completed sleep diary to the study investigator at that appointment, after which they began home CPAP therapy.
CPAP treatment was prescribed and initiated by the sleep center’s provider. All participants received standardized patient education about OSA and CPAP treatment prior to initiating CPAP treatment at home. This included receiving a pamphlet about OSA and CPAP treatment from the sleep center, viewing a 15-minute video about OSA and CPAP at the sleep center prior to diagnostic and CPAP titration polysomnograms, and CPAP use/trouble-shooting instructions from the home medical equipment company at the time of CPAP set-up for home treatment. After one month of home CPAP treatment, participants returned to the sleep center for a research visit, and the objective CPAP use record was downloaded from the CPAP device (Figure 1).
Figure 1.
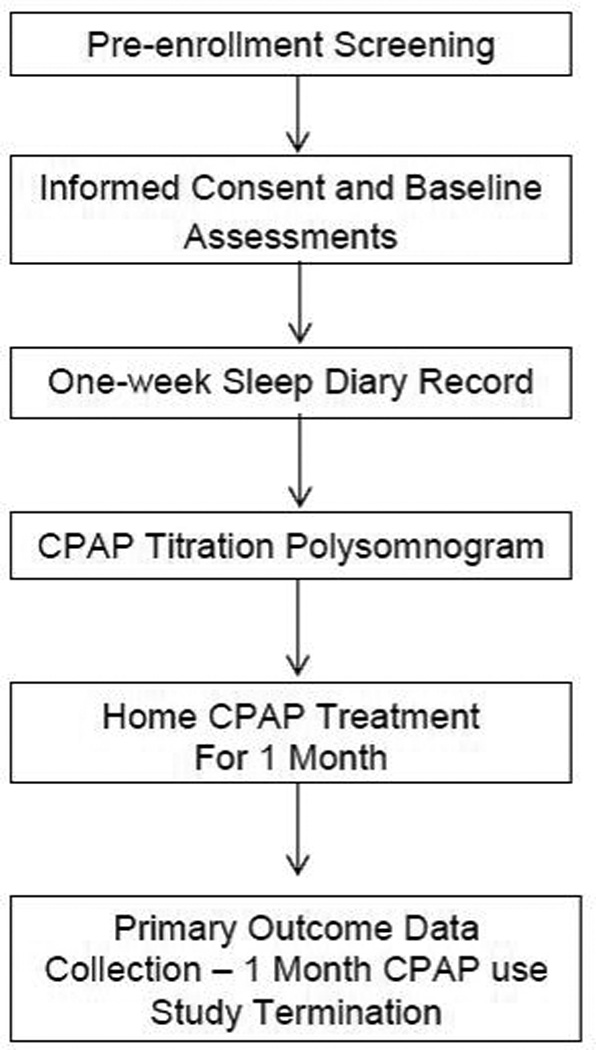
Study protocol
Notes. Abbreviations—CPAP, continuous positive airway pressure
Analysis
All variables were summarized using standard descriptive statistics and transformed if non-normality was identified. Bedtime variability was derived from sleep diaries of no less than five nights of complete data, and was calculated as the standard deviation in bedtime (minutes). Standard deviation was determined to be the most useful indicator of variance, as the unit of measure remains as minutes and is readily interpreted and clinically meaningful. Bedtime variability was not normally distributed, as was expected with a time-based variable, and the natural logarithmic transformation was used to improve the asymptotic normality of the distribution when evaluated as a continuous measure. Bedtime variability was also examined as an ordinal variable in increments of 15 minutes (0–15 minutes; 16–30, 31–15, 45–60, etc.) for the purposes of defining the amount of bedtime variability that may be clinically meaningful as a predictor of CPAP use, given lack of prior reports of the relationship of bedtime variability to CPAP non-adherence.
Two-sample t-tests on geometric means of transformed variables were used to determine whether differences in bedtime variability between adherent and non-adherent participants were present at one week of CPAP use and one month of CPAP use. Multivariable logistic regression was used to determine whether bedtime variability was an independent predictor of CPAP non-adherence and to identify increments of bedtime variability that influenced CPAP non-adherence.
Due to the limited sample size, five covariates were examined at a time in manual backwards selection logistic regressions for the outcomes of one-month and one-week CPAP use. An a priori decision was made to include covariates in further analyses if p ≤ 0.10. Marital status was the only covariate that met this criterion.
To explore potential moderators, separate multivariable logistic regression models with nested effects were examined for gender and education (as a proxy for socioeconomic status). In each multivariable logistic regression model for non-adherence, bedtime variability was nested within each level of the moderating variable, while accounting for marital status. This approach allowed the effect of bedtime variability on non-adherence to be interpreted separately for males and females, and similarly for the different levels of education.
Results
Sample Description and Group Comparison
The study sample (N=79) were predominantly white non-Hispanic, college-educated, obese, and employed full-time, with slightly more males than females. Most (68%) had been referred The majority had severe OSA (Table 1). Participants’ CPAP use averaged 4.6 hours/night (SD 2.5) at one week, with 27 (34.2%) below the minimal adherence level of 4 hours/night. The sample averaged 4.3 hours/night (SD 2.4) of CPAP at one month, with 31 (39.2%) below the 4-hour minimum for adherence. Hours of CPAP use per night were significantly different between CPAP adherents and non-adherents at both time points. There were no differences between one-month CPAP adherents and non-adherents on any baseline characteristic except marital status.
Table 1.
Characteristics of Total Sample and Those Non-Adherent and Adherent to CPAP Use ≥4 Hours/Night at One Month of Treatment
| Characteristic | Total (N=79) | Non-Adherent(n=31) | Adherent (n=48) | Difference(p) | |||
|---|---|---|---|---|---|---|---|
| n | % | n | % | n | % | ||
| Male | 43 | (54.4) | 13 | (41.9) | 30 | (62.5) | 0.07a |
| Race | |||||||
| White | 67 | (84.8) | 27 | (87.1) | 40 | (83.3) | 0.27b |
| Black or African American | 6 | (7.6) | 1 | (3.2) | 5 | (10.4) | |
| Native Hawaiian/ Pacific Islander | 2 | (2.5) | 2 | (6.5) | 0 | (0.0) | |
| Asian | 1 | (1.3) | 0 | (0.0) | 1 | (2.1) | |
| American Indian/Alaska Native | 3 | (3.8) | 1 | (3.2) | 2 | (4.2) | |
| Ethnicity | |||||||
| Hispanic or Latino | 7 | (8.9) | 5 | (16.1) | 2 | (4.2) | 0.10b |
| Not Hispanic or Latino | 72 | (91.1) | 26 | (83.9) | 46 | (95.8) | |
| Married | 54 | (68.4) | 15 | (48.4) | 39 | (81.2) | 0.002a |
| Education | |||||||
| High School | 31 | (39.2) | 15 | (48.4) | 16 | (51.6) | 0.18a |
| Some College or more | 48 | (60.8) | 16 | (33.3) | 32 | (66.7) | |
| Employment | |||||||
| Working Full-time | 49 | (62.0) | 16 | (51.6) | 33 | (68.8) | 0.82b |
| Working Part-time | 8 | (10.1) | 4 | (12.9) | 4 | (8.3) | |
| Homekeeper | 2 | (2.5) | 1 | (3.2) | 1 | (2.1) | |
| Unemployed | 6 | (7.6) | 3 | (9.7) | 3 | (6.3) | |
| Student | 2 | (2.5) | 1 | (3.2) | 1 | (2.1) | |
| Retired | 12 | (15) | 6 | (19.4) | 6 | (12.5) | |
| Rotating Shift Work | 5 | (6.3) | 4 | (12.9) | 1 | (2.1) | 0.07b |
| Night Shift Work (consistent shift) | 3 | (3.8) | 3 | (9.7) | 0 | (0.0) | 0.06b |
| Referral Source | |||||||
| Family/Primary Care Provider | 54 | (68) | 20 | (64.5) | 34 | (70.8) | 0.24b |
| Pulmonary Specialist | 12 | (15) | 5 | (16.1) | 7 | (14.6) | |
| Cardiology | 4 | (5.1) | 2 | (6.5) | 2 | (4.2) | |
| Neurology | 3 | (3.8) | 3 | (9.7) | 0 | (0.0) | |
| Family member or friend | 3 | (3.8) | 1 | (3.2) | 2 | (4.2) | |
| Self | 3 | (3.8) | 0 | (0.0) | 3 | (6.3) | |
| Apnea Hypopnea Index (events/hour) | |||||||
| Mild (5–15 events/hour) | 9 | (11.4) | 2 | (6.5) | 7 | (14.6) | 0.27c |
| Moderate (>15–30) | 21 | (26.6) | 8 | (25.8) | 13 | (27.1) | |
| Severe (>30) | 49 | (62.0) | 21 | (67.7) | 28 | (58.3) | |
| Mean | SD | Mean | SD | Mean | SD | ||
| Age (yrs) | 50.0 | (11.7) | 49.0 | (11.6) | 51.0 | (11.8) | 0.61d |
| Body Mass Index (kg/m2) | 38.1 | (8.6) | 39.1 | (9.7) | 37.4 | (7.9) | 0.40d |
| Apnea Hypopnea Index (events/hour) | 37.8 | (20.7) | 39.9 | (20.5) | 36.4 | (20.9) | 0.47d |
| NR Oxyhemoglobin Saturation Nadir | 80.8 | (7.1) | 80.8 | (7.8) | 80.8 | (6.6) | 0.98d |
| CPAP Use at 7 days (mean hours/night) | 4.6 | (2.5) | 2.1 | (1.8) | 6.2 | (1.4) | <0.001d |
| CPAP Use at 30 days (mean hours/night) | 4.3 | (2.4) | 1.7 | (1.3) | 5.9 | (1.1) | <0.001d |
Note. CPAP= Continuous Positive Airway Pressure; SD= standard deviation; NR= Non-Rapid Eye Movement.
chi-square
Fisher’s exact test
Cochran-Mantel-Haenszel
t-test
Pre-treatment Bedtime Variability and CPAP Non-adherence at One Week
Mean pre-treatment bedtime variability was 63.10 minutes (SD 38.04; Median 55.93, Interquartile Range [IQR] 38.17). Pre-treatment bedtime variability was not statistically different between CPAP adherents and non-adherents at one week (Table 2) and did not predict CPAP non-adherence at one week in a multivariable logistic regression model in which bedtime variability was the independent variable and marital status was included as a covariate (Table 3). The wide confidence intervals (i.e., precision of effect) were consistent with limited power to detect a small effect.
Table 2.
Pre-treatment Bedtime Variability of Participants who were Adherent and Non-adherent to CPAP Use ≥4 Hours per Night
| Time | Adherence | n | Bedtime Variability(minutes)a |
Coefficient of Variation |
pb |
|---|---|---|---|---|---|
| One Week | Adherent | 52 | 54.6 | 0.4 | 0.39 |
| Non-adherent | 27 | 60.3 | 0.5 | ||
| One Month | Adherent | 48 | 49.4 | 0.5 | 0.02 |
| Non-adherent | 31 | 66.7 | 0.4 |
Note. CPAP, Continuous Positive Airway Pressure; Adherent = ≥ 4hrs/night CPAP use; Non-adherent = < 4hrs/night CPAP use
Geometricmean of log-transformed data
Two-sample Satterthwaitet-test
Table 3.
Pre-treatment Bedtime Variability as Predictor of CPAP Non-Adherence (<4 Hours/Night) inMultivariate Logistic Regression(N=79)
| Time | Variable | B Estimate | Standard Error | Χ2 | p |
|---|---|---|---|---|---|
| One Weeka | Bedtime Variability (ln)a | 0.20 | 0.54 | 0.15 | 0.700 |
| Single Marital Statusb | 0.68 | 0.26 | 6.89 | 0.009 | |
| One Monthb | Bedtime Variability (ln)c | 1.24 | 0.59 | 4.38 | 0.036 |
| Single Marital Statusd | 0.73 | 0.27 | 7.53 | 0.006 |
Notes. CPAP= Continuous Positive Airway Pressure; ln= natural log
Adjusted OR=1.23, 95% CI 0.43, 3.51
Adjusted OR=3.90, 95% CI 1.41,10.77
Adjusted OR=3.45, 95% CI 1.08, 11.02
Adjusted OR=4.33, 95% CI 1.52 12.31
Pre-treatment Bedtime Variability and CPAP Non-adherence at One Month
The 31 participants who were non-adherent at one month had significantly greater variability in pre-treatment bedtime (Table 2). In a multivariable logistic regression model in which pre-treatment bedtime variability was the independent variable and marital status was included as a covariate, both pre-treatment bedtime variability and marital status independently predicted one-month CPAP non-adherence (Table 3). At one month of treatment, the adjusted odds of using CPAP an average of less than 4 hours/night were 3.5 times greater for each increment of pre-treatment bedtime variability and 4.3 times greater for unmarried than for married participants. Again, wide confidence intervals were seen.
To explore how much bedtime variability was important, a series of multivariable logistic regression analyses (not shown) were conducted to determine the specific level of pre-treatment bedtime variability that predicted non-adherence at one month. A separate analysis was done to evaluate each 15-minute increment as a cut-off point, each entered along with the covariate of marital status as an independent variable of bedtime variability ≥15 minutes (yes/no), ≥30 minutes (yes/no), etc. The adjusted odds of CPAP non-adherence at one month were 3.1 times greater for those whose bedtime varied by 75 minutes or more (95% CI 1.0 – 9.3; p=0.0495). Marital status was also an independent predictor of CPAP non-adherence (adjusted OR 4.5; 95% CI 1.6–12.8; p=0.005). Bedtime variability of 60 minutes or less did not predict non-adherence (p=0.37) when adjusted for marital status.
Exploratory Analysis of Potential Moderating Factors
Men who were non-adherent to CPAP had greater bedtime variability than did adherent men when compared using t-tests, while for female participants, the difference in bedtime variability between non-adherents and adherents was not statistically significant (Table 4). Likewise, in multivariable logistic regression models with nested effects, accounting for marital status, pre-treatment bedtime variability had a modest significant effect on adherence for males (OR=exp(1.78)=5.9, 95% CI = 1.2–30.7; p=0.03) but not for females (OR=exp(0.60)=1.8, 95% CI= 0.3–11.6; p=0.53), although significance was likely limited by lack of statistical power.
Table 4.
Differences in Bedtime Variability by Gender and One-Month CPAP Non-adherence (<4 Hours/Night)
| Gender | Adherence | n | Bedtime Variability(ln)a | Coefficient of Variation |
Pb |
|---|---|---|---|---|---|
| Male | Adherent | 36 | 49.40 | 0.45 | 0.02 |
| Non-adherent | 17 | 73.70 | 0.59 | ||
| Female | Adherent | 23 | 51.42 | 0.37 | 0.25 |
| Nonadherent | 21 | 60.34 | 0.43 | ||
Note. CPAP,=Continuous Positive Airway Pressure; ln = natural log
Geometric mean
Two-sample t-test applied to natural log-transformed minutes of bedtime variability
Education (as proxy for socioeconomic status) was examined as a potential modifier of the effect of bedtime variability on CPAP non-adherence at one month. Highest education completed was rank-ordered as junior high school and high school (n=34), some college (n=34), and four years or more of college (n=29). In a multivariable logistic regression model with nested effects wherein marital status was accounted for, in adults at the lowest education level, an increment of bedtime variability increased the odds of CPAP non-adherence 11-fold (adjusted OR=exp(2.4)=11, 95% CI 1.4–82.3; p=0.02). Similarly, when the subgroup with bedtime variability of ≥ 75 minutes was compared to those with less variability, the adjusted odds of non-adherence at one month were 18 times greater for those with lower education (OR=exp(2.9)=18, 95% CI 1.8–199.0; p=0.01) but not in those with some college education or four years of college or more (p=0.82 and p=0.55, respectively). Results were supported when frequency counts of one-month CPAP non-adherence and bedtime variability ≥ 75 minutes were compared in participants with junior high or high school completion, some college, and four years or more of college (Fisher’s exact tests p=0.006, p=1.0, p=0.6, respectively).
Discussion
These results suggest that pre-treatment bedtime variability, or inconsistency in time of going to bed to initiate sleep, is an important and modifiable predictor of one-month CPAP non-adherence, especially among men and those with little completed education. More than 75 minutes of bedtime variability conferred significantly more statistical risk for CPAP non-adherence than having less than 75 minutes of bedtime variability. These findings provide preliminary evidence to support the inclusion of sleep schedule stabilization, or minimizing bedtime inconsistency, in future interventions aimed at reducing CPAP non-adherence.
Smith and colleagues (2009) examined the effect of a patient education intervention based, in part, on the concepts of habit formation and consistency of routine. This intervention was rooted in Triandis’ Theory of Behavior (Triandis, 1982), in which repeated behaviors become habitual and support the adoption of a health behavior. In their small randomized controlled trial, the intervention group had significantly higher frequency of at least four hours per night of CPAP use at one month (p<0.01). Although the intervention was multi-factorial and did not specifically include bedtime variability as an intervention target, habit formation may be an important intervention focus for the promotion of CPAP adherence. Based on our findings and these theoretical assumptions about habit formation and health behavior, interventions to support the habitual behavior of going to bed at a more consistent time may support the routine application and use of CPAP among adults with OSA.
Although our results are preliminary, males and those with lower education had greater risk for bedtime variability and CPAP non-adherence. Recent evidence also supports gender and socioeconomic differences in adherence to CPAP treatment (Billings et al., 2011; Guralnick et al., 2012; Platt et al., 2009; Ye et al., 2012). Lower socioeconomic status and male gender may also be linked to prolonged sleep latency and difficulty initiating sleep (Grandner et al., 2010). Addressing bedtime variability in these groups may reduce their disproportionate rates of CPAP non-adherence.
Whether bedtime variability is a function of lifestyle, daily demands, or a symptom expression of overlapping insomnia (Luyster et al., 2010) cannot be determined based on the current study. Co-existent insomnia is prevalent in adults with OSA (Luyster, Buysse, & Strollo, 2010), and we did not specifically exclude those with insomnia or other sleep disorders, but no participants had diagnoses of insomnia that could be discerned from medical records. In future studies of treatment non-adherence, purposefully including subgroups with and without comorbid insomnia and OSA may address how sleep patterns and habits influence CPAP non-adherence in these commonly overlapping sleep disorders. Bedtime variability and other sleep patterns such as early morning awakenings and prolonged sleep latency are inherent in insomnia, and study of a broader set of sleep schedule factors may be important to understand CPAP non-adherence. Researchers may consider sleep schedule as a target of cognitive behavioral therapy to address both self-management of insomnia and CPAP adherence. We did not exclude shift workers from study participation, but representation of shift workers in our sample was modest (n=5). Rotating shift work in our study was not associated with bedtime variability, but greater representation of shift workers in the sample is needed to answer this question.
A limitation of the study was the use of sleep diaries, a self-report, subjective measure of the primary variable of interest of bedtime variability. Participants were provided with standardized instructions for completion of the diaries by a researcher to minimize the risk of systematic error in diary data. Diary forms also included completion instructions including how and when diaries were to be completed to reduce recall bias. A second limitation was the relatively short diary timespan. A one-week period of measuring bedtime variability may not be representative of long-term patterns of bedtime variability. We also did not know whether the diary week was representative of a participant-defined normal week of bedtimes.
Future studies of bedtime variability might include concurrent activity assessment with actigraphy, to objectively delineate pre-bedtime activity levels, bedtime, sleep onset, wake after sleep onset, and early morning awakenings. These additional sleep schedule variables would permit a more comprehensive analysis of how sleep patterns and sleep routines contribute to CPAP non-adherence. In addition, a more detailed record of pre-bedtime activities, bedtime routines, and sleep patterns may give more insight into bedtime variability and routines. Weekday and weekend day sleep schedule variables may also be examined in future work, to further elicit important differences in sleep schedule that may be amenable to behavioral interventions to reduce non-adherence. This likely necessitates a longer interval of assessment of diary data than the current study employed but may be facilitated by data collection methods such as ecological momentary assessments and other mobile technologies (Shiffman, Stone, & Hufford, 2008).
Although our findings are preliminary, bedtime variability appears to be an important modifiable factor for minimizing CPAP non-adherence that, to our knowledge, has not been reported previously. Based on our findings and those on adherence to medication, habitual behaviors are a line of inquiry likely to shed light on the problem of poor adherence to CPAP. Future prospective, longitudinal studies of CPAP adherence should include larger samples and more objective and detailed assessment of bedtime and sleep, distinguishing bedtime and sleep onset time as well as pre-sleep routines/activities and consistency of awake and out-of-bed times. Future intervention studies might include pre-treatment bedtime as a modifiable behavior to improve CPAP non-adherence outcomes in adults with OSA.
Acknowledgements
The research was funded by National Institutes of Health/National Institute for Nursing Research (NINR; K99NR011173, Sawyer, Principal Investigator; and R00NR011173, Sawyer, Principal Investigator). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institute of Nursing Research or National Institutes of Health. The investigators extend their appreciation to the clinical sleep center staffs at Penn State Hershey Medical Center and Lung Health Sleep Enhancement Specialists. We also acknowledge the contributions of Leon Sweer, MD, who served as a clinical site director for the study.
Contributor Information
Amy M. Sawyer, Email: ams24@psu.edu, Assistant Professor, Pennsylvania State University College of Nursing, 201 HHD East, University Park, Pennsylvania 16802.
Tonya S. King, Department of Public Health Sciences, PennState Hershey College of Medicine, Hershey, Pennsylvania
Douglas A. Sawyer, Pennsylvania State University College of Nursing, University Park, Pennsylvania
Albert Rizzo, Christiana Care Health System, Pulmonary Associates, P.A., Newark, Delaware.
References
- Aloia MS, Arnedt JT, Stanchina M, Millman RP. How early in treatment is PAP adherence established? Revisiting night-to-night variability. Behavioral Sleep Medicine. 2007;5:229–240. doi: 10.1080/15402000701264005. [DOI] [PubMed] [Google Scholar]
- Billings ME, Auckley D, Benca R, Foldvary-Schaefer N, Iber C, Redline S, Kapur VK. Race and residential socioeconomics as predictors of CPAP adherence. Sleep. 2011;34:1653–1658. doi: 10.5665/sleep.1428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Budhiraja R, Parthasarathy S, Drake CL, Roth T, Sharief I, Budhiraja P, Saunders V. Early CPAP use identifies subsequent adherence to CPAP therapy. Sleep. 2007;30:320–324. [PubMed] [Google Scholar]
- Centers for Medicare and Medicaid Services. Continuous positive airway pressure (CPAP) therapy for obstructive sleep apnea (OSA) Centers for Medicare and Medicaid Services; 2008. http://www.cms.gov/Outreach-and-Education/Medicare-Learning-Network-MLN/MLNMattersArticles/downloads/mm6048.pdf: [Google Scholar]
- Crawford MR, Espie CA, Bartlett DJ, Grunstein RR. Integrating psychology and medicine in CPAP adherence--new concepts? Sleep Medicine Reviews. 2014;18:123–139. doi: 10.1016/j.smrv.2013.03.002. [DOI] [PubMed] [Google Scholar]
- Grandner MA, Patel NP, Gehrman PR, Xie D, Sha D, Weaver T, Gooneratne N. Who gets the best sleep? Ethnic and socioeconomic factors related to sleep complaints. Sleep Medicine. 2010;11:470–478. doi: 10.1016/j.sleep.2009.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guralnick AS, Pant M, Minhaj M, Sweitzer BJ, Mokhlesi B. CPAP adherence in patients with newly diagnosed obstructive sleep apnea prior to elective surgery. Journal of Clinical Sleep Medicine. 2012;8:501–506. doi: 10.5664/jcsm.2140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoenig JM, Heisey DM. The abuse of power: The pervasive fallacy of power calculations for data analysis. The American Statistician. 2001;55:19–24. [Google Scholar]
- Iber C, Ancoli-Israel S, Chesson A, Quan SF. The AASM manual for the scoring of sleep and associated events. Westchester, IL: American Academy of Sleep Medicine; 2007. [Google Scholar]
- Luyster F, Buysse D, Strollo PJ., Jr Comorbid insomnia and obstructive sleep apnea: Challenges for clinical practice and research. Journal of Clinical Sleep Medicine. 2010;6:196–204. [PMC free article] [PubMed] [Google Scholar]
- McDonald HP, Garg AX, Haynes RB. Interventions to enhance patient adherence to medication prescriptions: Scientific review. Journal of American Medical Association. 2002;288:2868–2879. doi: 10.1001/jama.288.22.2868. [DOI] [PubMed] [Google Scholar]
- Platt AB, Field SH, Asch DA, Chen Z, Patel NP, Gupta R, Kuna ST. Neighborhood of residence is associated with daily adherence to CPAP therapy. Sleep. 2009;32:799–806. doi: 10.1093/sleep/32.6.799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quan SF, Awad KM, Budhiraja R, Parthasarathy S. The quest to improve CPAP adherence-- PAP potpourri is not the answer. Journal of Clinical Sleep Medicine. 2012;8:49–50. doi: 10.5664/jcsm.1660. doi: http://dx.doi.org/10.5664/jcsm.1936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sawyer AM, Deatrick J, Kuna ST, Weaver TE. Differences in perceptions of the diagnosis and treatment of obstructive sleep apnea and continuous positive airway pressure therapy among adherers and nonadherers. Qualitative Health Research. 2010;20:873–892. doi: 10.1177/1049732310365502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sawyer AM, Gooneratne NS, Marcus CL, Ofer D, Richards KC, Weaver TE. A systematic review of CPAP adherence across age groups: Clinical and empiric insights for developing CPAP adherence interventions. Sleep Medicine Reviews. 2011;15:343–356. doi: 10.1016/j.smrv.2011.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sawyer AM, King TS, Hanlon A, Richards KC, Sweer L, Rizzo A, Weaver TE. Risk assessment for CPAP nonadherence in adults with newly diagnosed obstructive sleep apnea: Preliminary testing of the Index for Nonadherence to PAP (I-NAP) Sleep & Breathing. 2014 doi: 10.1007/s11325-014-0959-z. Advnace online publication. doi: http://dx.doi.org/10.1007/s11325-014-0959-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schlenk EA, Burke LE, Rand C. Behavioral strategies to improve medication-taking compliance. In: Burke LE, Ockene IS, editors. Compliance in healthcare and research. New York: Futura Publishing Company, Inc.; 2001. pp. 57–70. [Google Scholar]
- Shiffman S, Stone AA, Hufford MR. Ecological momentary assessment. Annual Review of Clinical Psychology. 2008;4:1–32. doi: 10.1146/annurev.clinpsy.3.022806.091415. [DOI] [PubMed] [Google Scholar]
- Smith CE, Dauz E, Clements F, Werkowitch M, Whitman R. Patient education combined in a music and habit-forming intervention for adherence to continuous positive airway (CPAP) prescribed for sleep apnea. Patient Education and Counseling. 2009;74:184–190. doi: 10.1016/j.pec.2008.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Triandis HC. A model of choice. In: McAlister L, editor. Choice models for behavior. Greenwich: JAI Press, Inc.; 1982. pp. 147–163. [Google Scholar]
- Walters SJ. Consultants' forum: Should post-hoc sample size calculations be done? Pharmaceutical Statistics. 2009;8:163–169. doi: 10.1002/pst.334. [DOI] [PubMed] [Google Scholar]
- Weaver TE. Don't start celebrating -- CPAP adherence remains a problem. Journal of Clinical Sleep Medicine. 2013;9:551–552. doi: 10.5664/jcsm.2746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weaver TE, Grunstein RR. Adherence to continuous positive airway pressure therapy: The challenge to effective treatment. Proceedings of the American Thoracic Society. 2008;5:173–178. doi: 10.1513/pats.200708-119MG. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weaver TE, Kribbs NB, Pack AI, Kline LR, Chugh DK, Maislin G, Dinges DF. Night-to-night variability in CPAP use over first three months of treatment. Sleep. 1997;20:278–283. doi: 10.1093/sleep/20.4.278. [DOI] [PubMed] [Google Scholar]
- Ye L, Pack AI, Maislin G, Dinges D, Hurley S, McCloskey S, Weaver TE. Predictors of continuous positive airway pressure use during the first week of treatment. Journal of Sleep Research. 2012;21:419–426. doi: 10.1111/j.1365-2869.2011.00969.x. [DOI] [PMC free article] [PubMed] [Google Scholar]


