Abstract
Indoor air pollution (IAP) caused by cooking has been associated with lung cancer risk in retrospective case-control studies in developing and rural countries. We report the association of cooking conditions, fuel use, oil use and risk of lung cancer in a developed urban population in a prospective cohort of women in Shanghai. A total of 71,320 never smoking women were followed from 1996 through 2009 and 429 incident lung cancer cases were identified. Questionnaires collected information on household living and cooking practices for the women’s three most recent residences and utilization of cooking fuel and oil, and ventilation conditions. Cox proportional hazards regression estimated the association for kitchen ventilation conditions, cooking fuels, and use of cooking oils for the risk of lung cancer by hazard ratios (HR) with 95% confidence intervals (95% CI). Ever poor kitchen ventilation was associated with a 49% increase in lung cancer risk (HR: 1.49; 95% CI: 1.15–1.95) compared to never poor ventilation. Ever use of coal was not significantly associated. However, ever coal use with poor ventilation (HR: 1.69; 95% CI: 1.22–2.35) and twenty or more years of using coal (HR: 2.03; 95% CI: 1.35–3.05) was significantly associated compared to no exposure to coal or poor ventilation. Cooking oil use was not significantly associated. These results demonstrate that IAP from poor ventilation of coal combustion increases the risk of lung cancer and is an important public health issue in cities across China where people may have lived in homes with inadequate kitchen ventilation.
Keywords: Ventilation, coal, lung cancer, never smoking, women, China, Shanghai
Introduction
In China, the estimated age-adjusted death-rate of lung cancer was 33.5 per 100,000 in 2008 according to a WHO report1. Lung cancer incidence has been on the rise in less-developed countries2. Approximately 18% of worldwide cancer deaths were lung cancer deaths, many of which are preventable3 and largely attributable to tobacco use4. However, Asian women have a relatively high lung cancer rate compared to women of other ethnicities, despite low prevalence of smoking5.
Air pollution contributes to lung cancer risk, particularly in large urban cities where residential sources of air pollution contribute to poor air quality6. In particular, indoor air pollution (IAP) from coal use in homes is a lung cancer risk factor7, 8 and is an IARC class 1 carcinogen9. IAP is a major concern in less developed countries where biomass fuels are used for cooking and heating10. Indoor coal burning increases particulate matter (PM)11, polycyclic aromatic hydrocarbons (PAHs), and heterocyclic aromatic compounds12 in the air, which are associated with lung toxicity and cancer risk. Coal burning has been strongly associated with lung cancer risk in previous studies of rural and developing populations with high exposure13, 14 and poor home ventilation can increase exposure to carcinogenic particulates which elevates lung cancer risk8.
Besides coal, cooking oil emissions is another source of poor indoor air quality which may contain various genotoxins and mutagens15–19. Studies conducted in China and Taiwan have identified cooking oil fumes that contain PAHs in crudely refined cooking oils16, 18, 19. Some volatilized oils, such as olive and peanut, are low in PAHs compared to others such as soybean or rapeseed20. Trans-2,4-decadienal (tt-DDE), found in cooking fumes, can induce cell proliferation and cytokine production from oxidative stress21–23. Previous studies have suggested a dose-response relationship between lung cancer risk and years of cooking fumes exposure, particularly with high temperature frying which volatilizes cooking oils24, 25. Chinese-style cooking often involves volatilization of oils, potentially exposing people to more fumes than cooking methods from other regions of the world26.
Environmental tobacco smoke (ETS) has also been linked to lung cancer risk in non-smoking women27. Smoking prevalence in China is very high (>28%), so the likelihood of ETS exposure is very likely, particularly among middle-aged married women since the prevalence of smoking is above 60% in middle-aged men (45–64)28, and this exposure could lead to an elevation of lung cancer risk. Gene-environment interaction may make Asian non-smoking women susceptible to ETS exposure29.
Previous studies on fuel use, ventilation, indoor air quality, and lung cancer risk were case-control studies24, 30–37. However, few studies were conducted in urban areas where rapid socioeconomic development led to substantial modernization in kitchen ventilation and fuel use30, 31, 37. In contrast to previous studies, we assessed the association of indoor air quality and lung cancer risk due to cooking conditions and practices in a prospective, more developed, and urban population, the Shanghai Women’s Health Study cohort.
Materials and Methods
Study Population
The Shanghai Women’s Health Study cohort has been described in detail38. Briefly, a roster of 81,170 women, ages 40 to 70 years, was obtained from the resident offices in seven communities located in urban Shanghai, China. A total of 75,221 (92.7%) women participated in the study and completed baseline surveys between 1996 and 2000. Of those, we excluded 279 women who were found to be younger than age 40 years or older than age 70 years, 1,490 women who had prevalent cancer and 10 women who did not accrue any follow-up time, as well as 2043 women who were found to be ever smokers (1+ cigarette/day for 6+ months). The remaining cohort of 71,320 women was followed through December 2009 with in-person surveys and periodic linkage to cancer and vital statistics registries. A total of 429 lung cancer patients (ICD-9 Codes 162.1–162.9) was diagnosed during the follow-up period. All participants gave informed consent and the protocols used were approved by the institutional review boards of all institutions.
Data Collection and Follow-up
Information on household residences was collected in the baseline survey using a standardized and structured questionnaire with assistance and quality checks from a trained interviewer. Each subject was asked about each of the three most recent residences lived (duration), cooking fuel used (gas, coal, other), cooking oil used (soybean, vegetable, combination of soybean/vegetable, other), kitchen ventilation condition (good, fairly good, poor), smoking patterns at home (active smoking, husband smoking, other household residents), and coworker smoking habits at work (ever/never). From this information, years of use of each fuel and oil were calculated which covered approximately 75% of the cohort’s entire residential history. Additional information on employment and demographics was also collected at baseline.
The most recent follow-up occurred from 2007–2009. Newly diagnosed cancer cases were ascertained through biennial re-contact as well as record linkage to the Shanghai Cancer Registry; cancer registration is legally mandated in Shanghai, China. Information on date and hospital of diagnosis was collected. For patients whose diagnostic information could not be obtained from the cancer registry, medical charts and pathology slides were collected from diagnostic hospitals and reviewed by two study cancer pathologists to verify the diagnosis. In addition, the death certificate data from the Shanghai Vital Statistics Unit were collected to identify cause of death for deceased participants.
Statistical Analysis
Person-years for each subject were calculated from enrollment until lung cancer diagnosis, date of death, or the end of follow-up (December 31, 2009), whichever came first. The time scale was age at entry until end of study or loss to follow-up (censored) or event (lung cancer), and results are presented as hazard ratios (HR) with 95% confidence intervals (95% CI) from Cox proportional hazards regression to estimate the relative risk of lung cancer for ventilation, cooking fuel, or oil use categories. Kitchen ventilation condition (good/fairly good/poor), cooking fuel (gas, coal/coke balls), and oil use variables (vegetable, soybean, blend of soybean and vegetable, peanut) were calculated up through the most recent available information (baseline interview date for household conditions) and considered fixed through the end of follow-up.
Characteristics of the population were compared between ever and never poor ventilation using t-test for continuous and chi-square test for categorical variables. Kitchen ventilation (poor ventilation compared to good/fairly good ventilation), cooking fuel (coal compared to gas), and oil variables (vegetable compared to soybean, blend, peanut; soybean compared to vegetable, blend, peanut) were then individually evaluated in the models as the main effect for ever use, duration of time used, and time period of use. Also, we conducted a lagged analysis for the duration of time used by removing the most recent 10 years of exposure (effectively fixing exposures to 10 years prior to baseline) to identify if the most recent exposure contributed to lung cancer risks. In models that assessed both cooking fuel and ventilation conditions, the interaction term was the cross-product of the ventilation and coal variable. To test the proportional hazards assumption, we tested the interaction term for years of ventilation, years of fuel use, or years of cooking oil use and the natural log of age from enrollment; P-values were greater than 0.05, consistent with the assumption of proportional hazards. Each model was adjusted for age (continuous), education (elementary school, middle school, high school, college and beyond), family income (<10,000 yuan, 10,000–20,000 yuan, 20,000–30,000 yuan, >30,000 yuan), environmental tobacco smoke (ever/never), family history of lung cancer (yes/no), history of non-malignant lung disease (any one of asthma, bronchitis, tuberculosis), and occupational history (technical, governmental, administrative, manufacturing). Additionally, the possibility of a non-linear relationship between ventilation years and coal use years and lung cancer risk was assessed with the likelihood ratio test and restricted cubic splines utilizing the SAS LGTPHCURV9 macro by Li et al39. Five knots were fitted to the model. All significance tests were performed with a two-sided Wald chi-square at significance of 0.05. All analyses were performed in SAS 9.22 (SAS Institute, Inc. Cary, NC).
Results
The demographic characteristics of the Shanghai Women’s Health Study are presented in Table 1. Women who had poor ventilation were more likely to have been older, worked in a manufacturing occupation, have lower family income, be less educated, have no history of benign lung disease and be exposed to ETS (P-values < 0.0001). The majority of women in this cohort have cooked with coal in their lifetime, but women who ever lived in a home with poor ventilation were more likely to have used coal than women who never lived in a home with poor ventilation (84.9% versus 57.5%, P-value <0.0001). Family history of lung cancer and soybean oil use was not significantly different between people who ever had poor ventilation and those who never did. Few women (<3%) ever used vegetable oil for cooking, whereas nearly all women (>89%) had used soybean oil.
Table 1.
Distribution of fuel use and demographics by ventilation in the Shanghai Women’s Health Study, 1996–2000
| Ever Poor Ventilation
|
Never Poor Ventilation
|
P-value* | |||
|---|---|---|---|---|---|
| n=14069 | (%) | n=57251 | (%) | ||
| Age, years | |||||
| Mean, SD | 52.58 | (8.93) | 51.66 | (8.94) | <0.0001 |
| Follow-up Time, years | |||||
| Mean, SD | 10.93 | (1.56) | 11.06 | (1.45) | <0.0001 |
| Family History of Lung Cancer (1st degree relative) | |||||
| Yes | 207 | (1.47) | 768 | (1.34) | 0.23 |
| No | 13862 | (98.53) | 56483 | (98.66) | |
| Occupation | |||||
| Technical/Professional | 2810 | (20.05) | 15379 | (26.95) | <0.0001 |
| Government, Political, Legal | 421 | (3.05) | 2213 | (3.88) | |
| Administrative | 2953 | (21.05) | 11780 | (20.64) | |
| Manufacturing | 7825 | (55.85) | 27694 | (48.53) | |
| Family Income | |||||
| <10,000¥ | 2803 | (19.92) | 8375 | (14.63) | <0.0001 |
| 10,000–20,000¥ | 5875 | (41.76) | 21392 | (37.37) | |
| 20,000–30,000¥ | 3485 | (24.77) | 16707 | (29.18) | |
| >30,000¥ | 1902 | (13.52) | 10765 | (18.8) | |
| Education | |||||
| College | 1324 | (9.41) | 8520 | (14.88) | <0.0001 |
| High School | 3427 | (24.37) | 16768 | (29.29) | |
| Middle School | 5577 | (39.65) | 21157 | (36.96) | |
| Elementary School | 3737 | (26.57) | 10801 | (18.87) | |
| Ever Benign Lung Disease (Tuberculosis, Bronchitis, Asthma) | |||||
| Yes | 2001 | (12.97) | 7425 | (14.22) | <0.0001 |
| No | 12068 | (87.03) | 49826 | (85.78) | |
| Ever ETS (Home or Work) | |||||
| Yes | 10071 | (71.58) | 40238 | (70.28) | <0.0001 |
| No | 3998 | (28.41) | 17013 | (29.71) | |
| Gas Use | |||||
| Ever | 13834 | (98.33) | 56613 | (98.89) | <0.0001 |
| Never | 235 | (1.67) | 638 | (1.11) | |
| Coal Use | |||||
| Ever | 11946 | (84.91) | 32912 | (57.49) | <0.0001 |
| Never | 2123 | (15.09) | 24339 | (42.51) | |
| Soybean Oil Use | |||||
| Ever | 13733 | (97.61) | 55785 | (97.44) | 0.24 |
| Never | 336 | (2.39) | 1466 | (2.56) | |
| Vegetable Oil Use | |||||
| Ever | 1615 | (11.48) | 5180 | (9.05) | <0.0001 |
| Never | 12454 | (88.52) | 52071 | (90.95) | |
Abbreviations: SD, ETS; *Continuous: variables: t-test; Categorical variables: chi-square test
The associations of lung cancer risk with cooking coal use and kitchen ventilation are presented in Table 2. Ever having poor kitchen ventilation compared to never having poor kitchen ventilation was associated with a 49% increased risk of lung cancer (HR: 1.49; 95% CI: 1.15–1.95). Women who lived in a home with poor ventilation during both childhood and adulthood had a 69% elevated risk of lung cancer compared to women never exposed to poor ventilation during their lives (HR: 1.69; 95% CI: 1.19–2.42). Ever use of coal was not significantly associated with risk of lung cancer. However, with poor ventilation, exposure to coal (HR: 1.69; 95% CI: 1.22–2.35), particularly twenty or more years of using coal (HR: 2.03; 95% CI: 1.35–3.05), and exposure throughout childhood and adulthood (HR: 2.03; 95% CI: 1.34–3.07) was strongly associated with lung cancer risk compared to women never exposed to coal or poor ventilation. Additionally, 10 year lagged analyses and stratified analyses of kitchen ventilation and cooking fuel by ETS, occupation, income, education, and birth year did not show substantial heterogeneity (data not shown). Analyses of cooking oils were unremarkable as nearly all women used soybean oil and few women used vegetable oil; these results are presented in Supplemental Table 1.
Table 2.
Kitchen ventilation condition, coal use, and risk of lung cancer in the Shanghai Women’s Health Study
| Cases | Total N | HR* | 95% CI | |
|---|---|---|---|---|
| Poor Ventilation | ||||
| Never (Good/Fairly Good) | 351 | 57251 | 1.00 | (ref) |
| Ever | 78 | 14069 | 1.49 | (1.15–1.95) |
| >0–19.99 Years | 41 | 6618 | 1.54 | (1.09–2.16) |
| ≥20 Years | 37 | 7373 | 1.45 | (1.01–2.08) |
| Poor Ventilation Exposure Time Window | ||||
| Never Exposed (Good/Fairly Good) | 351 | 57251 | 1.00 | (ref) |
| Childhood Only (<20 years age) | 2 | 573 | 1.13 | (0.28–4.65) |
| Adulthood Only (≥20 years age) | 39 | 7746 | 1.37 | (0.97–1.92) |
| Childhood and Adulthood | 37 | 5750 | 1.69 | (1.19–2.42) |
| Coal Use | ||||
| Never (Gas Users) | 159 | 26462 | 1.00 | (ref) |
| Ever | 270 | 44858 | 1.03 | (0.84–1.26) |
| >0–19.99 Years | 165 | 26717 | 1 | (0.79–1.25) |
| ≥20 Years | 105 | 18141 | 1.09 | (0.85–1.40) |
| Coal Use Exposure Time Window | ||||
| Never Exposed (Gas Users) | 159 | 26462 | 1.00 | (ref) |
| Childhood Only (<20 years age) | 8 | 1941 | 1.56 | (0.76–3.19) |
| Adulthood Only (>20 years age) | 144 | 18712 | 1.01 | (0.80–1.28) |
| Childhood and Adulthood | 118 | 24205 | 1.04 | (0.81–1.33) |
| Coal Use With Poor Ventilation | ||||
| Never Coal (Gas User), Poor Ventilation (Good/Fairly Good) | 152 | 24339 | 1.00 | (ref) |
| Ever Coal or Poor Ventilation | 210 | 36141 | 0.97 | (0.77–1.22) |
| Ever Coal and Poor Ventilation | 67 | 10840 | 1.69 | (1.22–2.35) |
| >0–19.99 Years | 29 | 5483 | 1.39 | (0.89–2.17) |
| ≥20 Years | 38 | 5357 | 2.03 | (1.35–3.05) |
| Coal Use With Poor Ventilation Exposure Time Window | ||||
| Never Exposed (Gas User, Good/Fairly Good)) | 152 | 24339 | 1.00 | (ref) |
| Childhood Only (<20 years age) | 2 | 552 | 1.19 | (0.29–4.97) |
| Adulthood Only (≥20 years age) | 36 | 6147 | 1.54 | (1.04–2.30) |
| Childhood and Adulthood | 29 | 4141 | 2.03 | (1.34–3.07) |
Adjusted for age (continuous), education (elementary, middle, high, college), income (<10,000¥, 10,000–20,000¥, 20,000–30,000¥, >30,000¥), environmental tobacco (yes, no), family history of lung cancer (yes, no), history of non-malignant lung disease (yes, no), occupation (technical/professional, government/political, administrative, manufacturing) with additional mutual adjustment for models (eg. coal model adjusted for gas use, etc)
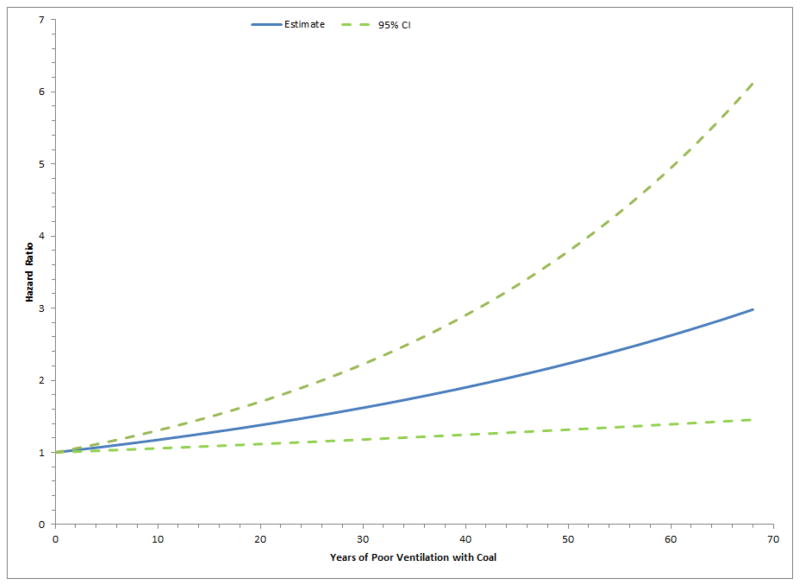
Figure 1 shows the effects of poorly ventilated coal years on lung cancer risk. The graph demonstrated a monotonic increase in the risk of lung cancer as the exposure years increased and the relationship appeared to be linear. The relationship did not achieve statistical significance for non-linearity (P-value: 0.97).
Figure 1.
Lung cancer hazard ratios by years of poorly ventilated coal use in the Shanghai Women’s Health Study. Model was adjusted for age (continuous), education (elementary, middle, high, college), environmental tobacco (yes, no), family history of lung cancer (yes, no), history of non-malignant lung disease (yes, no), occupation (technical/professional, government/political, administrative, manufacturing), vegetable oil use (yes/no). Test for non-linearity = 0.97.
Discussion
In this large prospective study of kitchen ventilation, cooking fuels, and cooking oils in never smoking women in Shanghai, coal use in poorly ventilated conditions was associated with an increased risk of lung cancer, and the strength of association increased with years of exposure. The association was strongest in women with both coal use and poor kitchen ventilation during both childhood and adulthood. Consistent with previous studies24, 30–37, poor indoor air quality was found to elevate lung cancer risk in our prospective cohort study. Conversely, we observed no elevated lung cancer risk associated with cooking oil use.
As development of urban areas in China continues, our data suggests indoor air conditions appear to be improving over time (63% of study population reported ever coal use and 20% of study population reported ever poor ventilation compared to 1% and 3% at baseline, respectively), but there may still be individuals living in Shanghai with current coal use and/or poor ventilation. In urban Shanghai, women with the least education and lowest family income were more likely to have lived in homes with poor ventilation and used coal for cooking (Spearman correlation: −0.12, P-value < 0.05) than more educated or higher income women. Therefore, in Shanghai as well as the rest of the developing China, efforts to reduce poverty could help improve cooking conditions in kitchens and reduce IAP and lung cancer risk.
In our study, over 70,000 women from various socioeconomic backgrounds were followed over time to estimate trends of coal use and home ventilation in the general population. We found that women who had poorly ventilated kitchens and used coal (about 60% of the study population ever used coal) for cooking had an increased risk of lung cancer. The strongest effects were observed in women exposed for more than 20 years and throughout both childhood and adulthood, suggesting longer duration of exposure increases the risk of lung cancer. Inadequate ventilation of kitchens could expose individuals to coal emissions containing carcinogens such as PAHs compounds12, 15–19. These PAHs can begin the process of carcinogenesis once activated by binding covalently to DNA, forming depurinating adducts and cause oxidative damage40. Genotoxicity resulting from PAH exposure is a gradual process with a long latency period prior to development of cancer41. Risk of lung cancer was highest in women with the longest duration of poor ventilation and coal use and most exposures began in earlier/older homes. Even women who ever but no longer used coal or had poor ventilation had an elevated risk of lung cancer compared to women with never exposure, resembling the elevated cancer risk pattern in former smokers42.
We also conducted stratified and restricted analyses by potential confounders such as occupation, ETS, income, and education to assess if they were concordant with kitchen ventilation or coal use patterns, which would explain the observed associations with lung cancer risk. Occupational exposures may influence lung cancer risk43. However, few women (~2.2%) in this population worked jobs (wood/paper and leather processing) that were significantly associated with elevated risk of lung cancer. We conducted a sensitivity analysis that excluded all manufacturing workers (49.8% of the women in this study) and the coal, ventilation, lung cancer risk associations did not change meaningfully. Stratifying analyses by occupational classification also did not reveal differential results by stratum (data not shown). Income and socioeconomic status could be concordant with coal use and kitchen ventilation conditions. Coal use and poor kitchen ventilation were negatively associated with income and education in linear regression models (adjusted for age, P-values < 0.0001). However, stratified analyses by income group did not reveal substantial heterogeneity in the effect estimates of coal use and poor kitchen ventilation on lung cancer risk. Further, excluding women in the lowest income group (<10,000 yuan) in a restricted analysis did not change the overall associations observed (data not shown). ETS was associated with lung cancer-related mortality in this cohort44. The effect estimates for ventilation and coal use did not differ more than 10% when adjusted by several ETS measures (eg. ever exposed to ETS, husband smoking status, years exposed to smoking at home), nor were the effect estimates substantially different from one another in stratified analyses by these ETS variables (ie. all P-interaction > 0.05). However, these ETS metrics may not fully capture all exposures to ETS, and there may still be some residual confounding. But ETS is unlikely to account for the entirety of the associations observed as ETS accounts for approximately a 24% elevation in lung cancer risk45. We also conducted analyses for each age group/cohort which did not reveal any differential effects across strata; poor kitchen ventilation and coal use were present across all age cohorts and the elevation of risk was consistent across all age groups.
To the best of our knowledge, this is the first prospective report of the association between kitchen ventilation and coal use with lung cancer risk in an urban city that has developed significantly over the past 20 years. Of the retrospective studies reporting indoor air quality and lung cancer risk in an urban population, one case-control study was conducted in the past 10 years in non-smoking women in Taiyuan, China which suggested an inverse association between kitchen ventilator use and the risk of lung cancer. However, Taiyuan was less developed than Shanghai and many women reported current exposure37, rather than past exposures as in our study. Other studies were either conducted before Shanghai underwent substantial development30 and/or had a case-control design30, 31.
The primary strengths of this study originate from the prospective nature of the Shanghai Women’s Health Study. This cohort had high participation rates (92%), and little loss to follow-up, minimizing biases inherent to case-control and retrospective studies (e.g. recall bias). Unique to this study population, the women in this study were non-smokers and lived in urban Shanghai, not rural regions of China where many of the previous studies of coal use and lung cancer risk were conducted.
We did not observe an association with cooking oils and lung cancer risk. Previous studies have identified strong associations between cooking oil and lung cancer risk25, 26, 31, 46, 47, and several studies suggested rapeseed oil24, 26, 30, 46, but this crudely refined oil has not been regularly used in the homes of women in this study for over 25 years. Modern cooking oils have become more refined and as a result, the genotoxic potential of these oils may be lower compared to crudely refined cooking oils identified in past studies which contained higher levels of PAH emissions when volatilized15. In epidemiologic studies, the measured effect between cooking oil and lung cancer risk has been modest (OR/RR generally less than 2.0), thus the effect would be less pronounced in populations where the prevalence of exposure is low (e.g. non-occupational settings). A significant limitation of the cooking oil analyses was the lack of detailed information on cooking methods, such as deep frying versus pan frying, frequency of cooking, smoked foods, and types of dishes prepared. Also, the data collection method for cooking oil was broad (e.g. vegetable, soybean) and lacked specificity (e.g. olive, grape seed, sesame). We did not conduct an analysis on peanut oil use as very few women (n=2021, cases=11) ever listed their predominant cooking oil as peanut.
As with most observational studies, there may be issues of exposure misclassification as the questionnaire assessment of kitchen ventilation was subjective. We conducted stratified analyses by factors that may influence the study observations and did not find heterogeneity suggestive of differential bias. Future studies could be improved with detailed questions to determine ventilation. Household variables were treated as fixed after the baseline interview which may lead to possible exposure misclassification if subjects moved or modified their kitchen after completing the baseline interview. However, any misclassification present is likely to be non-differential. A lagged analysis that removed the last 10 years of exposure did not change the associations observed (data not shown) as the vast majority of the exposures that contributed to lung cancer risk (poor ventilation and coal use) occurred prior to 10 years before baseline. About 75% of the cohort’s residential history was covered by the questionnaire, but about 25% was not. However, exclusion of those who did not have complete residential history did not substantially alter the results (<10% difference in effect estimates, data not shown). Lastly, information on household heating practices was not collected, so an analysis was not possible. However, most homes in Shanghai do not use household heating and would not likely alter the results.
In summary, this study conducted in a prospective cohort of women living in Shanghai suggests that exposure to coal fumes due to poor kitchen ventilation increases the risk of lung cancer. These findings were consistent with the notion that constituents of coal combustion increase the risk of lung cancer. Of note, this study observed that exposures which occurred many years in the past increased the risk of lung cancer. Because some homes in Shanghai and across China still have poor kitchen ventilation and use coal for cooking, intervention efforts directed at individuals likely to be exposed to cooking fumes (i.e. lowest socioeconomic status) may help improve indoor air quality and decrease lung cancer risk.
Supplementary Material
Novelty and Impact.
First study of home kitchen ventilation, cooking fuels, cooking oils, and lung cancer risk in a current, urban, and prospective population of China. Exposure to smoke from cooking with coal in poor ventilated conditions increased the risk of lung cancer.
Acknowledgments
This work supported by NIH intramural research program, NIH grant contract N02 CP1101066, NIH training grant T32-CA105666, and NIH research grant R01 CA70867.
Abbreviations used
- IAP
Indoor air pollution
- ETS
Environmental tobacco smoke
- HR
Hazard ratio
- CI
Confidence interval
- PAH
Polycyclic Aromatic Hydrocarbon
- PM
Particulate Matter
References
- 1.Organization WH. Disease and injury country estimates. 2012;2012 [Google Scholar]
- 2.Toh CK. The changing epidemiology of lung cancer. Methods Mol Biol. 2009;472:397–411. doi: 10.1007/978-1-60327-492-0_19. [DOI] [PubMed] [Google Scholar]
- 3.Organization WH. Global Health Observatory (GHO) Geneva, Switzerland: WHO; 2013. [Google Scholar]
- 4.Promotion NCfCDPaH; Prevention CfDCa, editor. Tobacco Use. Chronic Disease Prevention and Health Promotion; Atlanta, GA: 2011. [Google Scholar]
- 5.Epplein M, Schwartz SM, Potter JD, Weiss NS. Smoking-adjusted lung cancer incidence among Asian-Americans (United States) Cancer Cause Control. 2005;16:1085–90. doi: 10.1007/s10552-005-0330-6. [DOI] [PubMed] [Google Scholar]
- 6.Chan CK, Yao X. Air pollution in mega cities in China. Atmos Environ. 2008;42:1–42. [Google Scholar]
- 7.Hosgood HD, Wei H, Sapkota A, Choudhury I, Bruce N, Smith KR, Rothman N, Lan Q. Household coal use and lung cancer: systematic review and meta-analysis of case-control studies, with an emphasis on geographic variation. Int J Epidemiol. 2011;40:719–28. doi: 10.1093/ije/dyq259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lan Q, Chapman RS, Schreinemachers DM, Tian L, He X. Household stove improvement and risk of lung cancer in Xuanwei, China. J Natl Cancer Inst. 2002;94:826–35. doi: 10.1093/jnci/94.11.826. [DOI] [PubMed] [Google Scholar]
- 9.International Agency for Research on Cancer WHO. Household use of solid fuels and high-temperature frying. Lyon, France; Geneva: International Agency for Research on Cancer; 2010. p. vii.p. 424. Distributed by WHO Press. [Google Scholar]
- 10.Smith KR, Aggarwal AL, Dave RM. Air-Pollution and Rural Biomass Fuels in Developing-Countries - a Pilot Village Study in India and Implications for Research and Policy. Atmos Environ. 1983;17:2343–62. [Google Scholar]
- 11.Ezzati M, Zhou Z, Dionisio KL, Arku RE, Quaye A, Hughes AF, Vallarino J, Spengler JD, Hill A, Agyei-Mensah S. Household and community poverty, biomass use, and air pollution in Accra, Ghana. P Natl Acad Sci USA. 2011;108:11028–33. doi: 10.1073/pnas.1019183108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zhang JJ, Smith KR. Household air pollution from coal and biomass fuels in China: measurements, health impacts, and interventions. Environmental health perspectives. 2007;115:848–55. doi: 10.1289/ehp.9479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Armstrong B, Hutchinson E, Unwin J, Fletcher T. Lung cancer risk after exposure to polycyclic aromatic hydrocarbons: a review and meta-analysis. Environmental health perspectives. 2004;112:970–8. doi: 10.1289/ehp.6895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Barone-Adesi F, Chapman RS, Silverman DT, He X, Hu W, Vermeulen R, Ning B, Joseph F Fraumeni J, Rothman N, Lan Q. Risk of lung cancer associated with domestic use of coal in Xuanwei, China: retrospective cohort study. BMJ. 2012:345. doi: 10.1136/bmj.e5414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Shields PG, Xu GX, Blot WJ, Fraumeni JF, Jr, Trivers GE, Pellizzari ED, Qu YH, Gao YT, Harris CC. Mutagens from heated Chinese and U.S. cooking oils. J Natl Cancer Inst. 1995;87:836–41. doi: 10.1093/jnci/87.11.836. [DOI] [PubMed] [Google Scholar]
- 16.Chiang TA, Wu PF, Ko YC. Identification of carcinogens in cooking oil fumes. Environmental research. 1999;81:18–22. doi: 10.1006/enrs.1998.3876. [DOI] [PubMed] [Google Scholar]
- 17.Qu Y, Xu G, Zhou J, Chen T, Zhu L, Shields P, Wang H, Gao Y. Genotoxicity of heated cooking oil vapors. Mutation Research/Genetic Toxicology. 1992;298:105–11. doi: 10.1016/0165-1218(92)90035-x. [DOI] [PubMed] [Google Scholar]
- 18.Shuguang L, Dinhua P, Guoxiong W. Analysis of polycyclic aromatic hydrocarbons in cooking oil fumes. Archives of Environmental Health: An International Journal. 1994;49:119–22. doi: 10.1080/00039896.1994.9937464. [DOI] [PubMed] [Google Scholar]
- 19.Chiang TA, Wu PF, Wang LF, Lee H, Lee CH, Ko YC. Mutagenicity and polycyclic aromatic hydrocarbon content of fumes from heated cooking oils produced in Taiwan. Mutat Res. 1997;381:157–61. doi: 10.1016/s0027-5107(97)00163-2. [DOI] [PubMed] [Google Scholar]
- 20.Purcaro G, Navas JA, Guardiola F, Conte LS, Moret S. Polycyclic aromatic hydrocarbons in frying oils and snacks. J Food Prot. 2006;69:199–204. doi: 10.4315/0362-028x-69.1.199. [DOI] [PubMed] [Google Scholar]
- 21.Wang CK, Chang LW, Chang H, Yang CH, Tsai MH, Tsai HT, Lin P. Pulmonary changes induced by trans,trans-2,4-decadienal, a component of cooking oil fumes. Eur Respir J. 2010;35:667–75. doi: 10.1183/09031936.00140508. [DOI] [PubMed] [Google Scholar]
- 22.Young SC, Chang LW, Lee HL, Tsai LH, Liu YC, Lin P. DNA damages induced by trans, trans-2,4-decadienal (tt-DDE), a component of cooking oil fume, in human bronchial epithelial cells. Environ Mol Mutagen. 2010;51:315–21. doi: 10.1002/em.20550. [DOI] [PubMed] [Google Scholar]
- 23.Chang LW, Lo WS, Lin PP. Trans, trans-2,4-decadienal, a product found in cooking oil fumes, induces cell proliferation and cytokine production due to reactive oxygen species in human bronchial epithelial cells. Toxicol Sci. 2005;87:337–43. doi: 10.1093/toxsci/kfi258. [DOI] [PubMed] [Google Scholar]
- 24.Metayer C, Wang ZY, Kleinerman RA, Wang LD, Brenner AV, Cui HX, Cao JS, Lubin JH. Cooking oil fumes and risk of lung cancer in women in rural Gansu, China. Lung Cancer-J Iaslc. 2002;35:111–7. doi: 10.1016/s0169-5002(01)00412-3. [DOI] [PubMed] [Google Scholar]
- 25.Yu ITS, Chiu YL, Au JSK, Wong TW, Tang JL. Dose-response relationship between cooking fumes exposures and lung cancer among Chinese nonsmoking women. Cancer Res. 2006;66:4961–7. doi: 10.1158/0008-5472.CAN-05-2932. [DOI] [PubMed] [Google Scholar]
- 26.Zhong LJ, Goldberg MS, Gao YT, Jin F. Lung cancer and indoor air pollution arising from Chinese style cooking among nonsmoking women living in Shanghai, China. Epidemiology. 1999;10:488–94. [PubMed] [Google Scholar]
- 27.Fontham ETH, Correa P, Reynolds P, Wuwilliams A, Buffler PA, Greenberg RS, Chen VW, Alterman T, Boyd P, Austin DF, Liff J. Environmental Tobacco-Smoke and Lung-Cancer in Nonsmoking Women - a Multicenter Study. Jama-J Am Med Assoc. 1994;271:1752–9. [PubMed] [Google Scholar]
- 28.Li Q, Hsia J, Yang G. Prevalence of smoking in China in 2010. The New England journal of medicine. 2011;364:2469–70. doi: 10.1056/NEJMc1102459. [DOI] [PubMed] [Google Scholar]
- 29.Lan Q, Hsiung CA, Matsuo K, Hong YC, Seow A, Wang Z, Hosgood HD, 3rd, Chen K, Wang JC, Chatterjee N, Hu W, Wong MP, et al. Genome-wide association analysis identifies new lung cancer susceptibility loci in never-smoking women in Asia. Nat Genet. 2012;44:1330–5. doi: 10.1038/ng.2456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gao YT, Blot WJ, Zheng W, Ershow AG, Cheng WH, Levin LI, Rong Z, Fraumeni JF. Lung-Cancer among Chinese-Women. Int J Cancer. 1987;40:604–9. doi: 10.1002/ijc.2910400505. [DOI] [PubMed] [Google Scholar]
- 31.Ko YC, Lee CH, Chen MJ, Huang CC, Chang WY, Lin HJ, Wang HZ, Chang PY. Risk factors for primary lung cancer among non-smoking women in Taiwan. Int J Epidemiol. 1997;26:24–31. doi: 10.1093/ije/26.1.24. [DOI] [PubMed] [Google Scholar]
- 32.Wuwilliams AH, Dai XD, Blot W, Xu ZY, Sun XW, Xiao HP, Stone BJ, Yu SF, Feng YP, Ershow AG, Sun J, Fraumeni JF, et al. Lung-Cancer among Women in North-East China. Brit J Cancer. 1990;62:982–7. doi: 10.1038/bjc.1990.421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mumford JL, He XZ, Chapman RS, Cao SR, Harris DB, Li XM, Xian YL, Jiang WZ, Xu CW, Chuang JC, Wilson WE, Cooke M. Lung-Cancer and Indoor Air-Pollution in Xuan-Wei, China. Science. 1987;235:217–20. doi: 10.1126/science.3798109. [DOI] [PubMed] [Google Scholar]
- 34.Lissowska J, Bardin-Mikolajczak A, Fletcher T, Zaridze D, Szeszenia-Dabrowska N, Rudnai P, Fabianova E, Cassidy A, Mates D, Holcatova I, Vitova V, Janout V, et al. Lung cancer and indoor pollution from heating and cooking with solid fuels - The IARIC International Multicentre Case-Control study in Eastern/Central Europe and the United Kingdom. American Journal of Epidemiology. 2005;162:326–33. doi: 10.1093/aje/kwi204. [DOI] [PubMed] [Google Scholar]
- 35.Liu ZY, He XZ, Chapman RS. Smoking and Other Risk-Factors for Lung-Cancer in Xuanwei, China. Int J Epidemiol. 1991;20:26–31. doi: 10.1093/ije/20.1.26. [DOI] [PubMed] [Google Scholar]
- 36.Ramanakumar AV, Parent ME, Siemiatycki J. Risk of lung cancer from residential heating and cooking fuels in Montreal, Canada. American Journal of Epidemiology. 2007;165:634–42. doi: 10.1093/aje/kwk117. [DOI] [PubMed] [Google Scholar]
- 37.Mu LN, Liu L, Niu RG, Zhao BX, Shi JP, Li YL, Swanson M, Scheider W, Su J, Chang SC, Yu SZ, Zhang ZF. Indoor air pollution and risk of lung cancer among Chinese female non-smokers. Cancer Cause Control. 2013;24:439–50. doi: 10.1007/s10552-012-0130-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Zheng W, Chow WH, Yang G, Jin F, Rothman N, Blair A, Li HL, Wen W, Ji BT, Li Q, Shu XO, Gao YT. The Shanghai Women’s Health Study: rationale, study design, and baseline characteristics. Am J Epidemiol. 2005;162:1123–31. doi: 10.1093/aje/kwi322. [DOI] [PubMed] [Google Scholar]
- 39.Li R, Hertzmark E, Louie M, Chen L, Spiegelman D. The SAS LGTPHCURV9 Macro. Vol. 2013. Harvard School of Public Health; 2011. [Google Scholar]
- 40.Xue WL, Warshawsky D. Metabolic activation of polycyclic and heterocyclic aromatic hydrocarbons and DNA damage: A review. Toxicol Appl Pharm. 2005;206:73–93. doi: 10.1016/j.taap.2004.11.006. [DOI] [PubMed] [Google Scholar]
- 41.Harrison RM, Smith DJT, Kibble AJ. What is responsible for the carcinogenicity of PM2.5? Occupational and environmental medicine. 2004;61:799–805. doi: 10.1136/oem.2003.010504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Peto R, Darby S, Deo H, Silcocks P, Whitley E, Doll R. Smoking, smoking cessation, and lung cancer in the UK since 1950: combination of national statistics with two case-control studies. British medical journal. 2000;321:323–9. doi: 10.1136/bmj.321.7257.323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Pronk A, Coble J, Ji BT, Shu XO, Rothman N, Yang G, Gao YT, Zheng W, Chow WH. Occupational risk of lung cancer among lifetime non-smoking women in Shanghai, China. Occupational and environmental medicine. 2009;66:672–8. doi: 10.1136/oem.2008.043695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Wen WQ, Shu XO, Gao YT, Yang G, Li Q, Li HL, Zheng W. Environmental tobacco smoke and mortality in Chinese women who have never smoked: prospective cohort study. British medical journal. 2006;333:376–9. doi: 10.1136/bmj.38834.522894.2F. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Hackshaw AK, Law MR, Wald NJ. The accumulated evidence on lung cancer and environmental tobacco smoke. BMJ. 1997;315:980–8. doi: 10.1136/bmj.315.7114.980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ko YC, Cheng LSC, Lee CH, Huang JJ, Huang MS, Kao EL, Wang HZ, Lin HJ. Chinese food cooking and lung cancer in women nonsmokers. American Journal of Epidemiology. 2000;151:140–7. doi: 10.1093/oxfordjournals.aje.a010181. [DOI] [PubMed] [Google Scholar]
- 47.Seow A, Poh WT, Teh M, Eng P, Wang YT, Tan WC, Yu MC, Lee HP. Fumes from meat cooking and lung cancer risk in Chinese women. Cancer Epidem Biomar. 2000;9:1215–21. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.