Highlights
-
•
Differential responsiveness to early panocular atoh7 expression.
-
•
Early panocular atoh7 only mildly affects dorsal retina (reduced Müller glia cells).
-
•
Early panocular atoh7 severely affects ventral retina (predominant RGC fate).
-
•
Early panocular atoh7 expression results in coloboma.
Keywords: Atoh7 (Ath5), RPC, Retinal differentiation, Fate shift, Optic fissure, Coloboma
Abstract
During vertebrate eye development retinal progenitor cells (RPCs) differentiate into all neural cell types of the retina. Retinal ganglion cells (RGCs) represent the first cell type to be generated. For their development, Atoh7, a basic Helix Loop Helix (bHLH) transcription factor is crucial. Atoh7 loss of function results in a massive reduction or even a total loss of RGCs. However, inconsistent results have been obtained in atoh7 gain of function experiments with respect to ganglion cell genesis, implying that the effect of Atoh7 is likely to be dependent on the competence state of the RPC.
In this study we addressed the differential susceptibilities of early RPCs to Atoh7 in vivo, using medaka. Unexpectedly, we observed a largely normal development of the dorsal retina, although atoh7 was precociously expressed. However, the development of the retina close to the optic nerve head (part of the ventral retina) was disturbed severely. Photoreceptors were largely absent and the Müller glia cell number was reduced significantly. The majority of cells in this domain were ganglion cells and the abnormal development of this area affected the closure of the optic fissure resulting in coloboma.
1. Introduction
During early vertebrate eye development retinal progenitor cells (RPCs) of the eye field form the optic vesicle by single RPC outward migration (Rembold, 2006). Subsequently the optic vesicle is rearranged into the optic cup, containing a transient fissure at the ventral pole, the optic fissure (Li et al., 2000; Fuhrmann, 2010; Martinez-Morales and Wittbrodt, 2009; Sinn and Wittbrodt, 2013).
RPCs of the optic cup undergo a series of conserved competence states during their differentiation into six neuronal and one glial cell type (Wong and Rapaport, 2009; Matter-Sadzinski, 2005; Livesey and Cepko, 2001). The first cells to be generated after the onset of retinal differentiation are ganglion cells, retinal projection neurons, and the last cells to be generated are photoreceptor cells and Müller glia cells (Malicki, 2004; Livesey and Cepko, 2001; Cepko et al., 1996; Bassett and Wallace, 2012). In the differentiated retina the cell types are arranged in conserved layers, from basal to apical, the ganglion cell layer (GCL), the inner nuclear layer (INL, bipolar cells, Müller glia cells, amacrine cells, horizontal cells) and the outer nuclear layer (ONL, photoreceptor cells), separated by plexiform layers. In mammals RPCs express the retinal homeobox gene, e.g. Rax in mouse. However in teleosts three paralogs of Rax have been identified (rx 1, 2 and 3). In medaka (Oryzias latipes) rx2 is expressed in all RPCs starting at the optic vesicle stage and lasting until differentiation is initiated (Loosli et al., 2001). Importantly, while differentiation is active throughout the retina, the margins of the optic fissure are undifferentiated, marked by rx2 positive RPCs, in order to allow fissure fusion as development proceeds.
In the differentiated retina rx2 becomes restricted to retinal stem cells in the CMZ, Müller glia cells and photoreceptors. Differentiation of RPCs is initiated by differential expression of various transcription factors depending on the cell types generated (Run-Tao Yan, 2005; Matter-Sadzinski, 2005; Wang and Harris, 2005). The key factor marking the onset of retinal differentiation, and thereby the generation of retinal ganglion cells (RGCs) is the bHLH transcription factor, Atonal homolog seven (Atoh7, formerly Ath5) (Kay, 2005; Kay et al., 2001; Bassett and Wallace, 2012). Atoh7 is expressed in response to signals emanating from the optic stalk (Martinez-Morales et al., 2005; Masai et al., 2000) shortly after the expression of rx2 in RPCs of the central retina is declining (Loosli et al., 2001). A loss of function of atoh7 results in a complete loss of RGCs in zebrafish (Kay et al., 2001) and a massive reduction of RGCs in mouse (Le et al., 2006). The overexpression of atoh7 however has resulted in inconsistent findings, presumably due to different experimental conditions. Clonal overexpression of atoh7 resulted in favored ganglion cell differentiation at the expense of bipolar and Müller glia cells during clonal differentiation (Kanekar et al., 1997). However, since the outcome of atoh7 overexpression was analyzed clonally the total number of ganglion cells within the eye remains unclear. It is conceivable that the total number of ganglion cells is tightly regulated and hence not altered (Zhang and Yang, 2001; Gonzalez-Hoyuela et al., 2001).
Cell type specific overexpression of Atoh7 in predefined lineages (in mouse) resulted in divergent findings (Prasov and Glaser, 2012a; Mao et al., 2013). Atoh7 was shown to be capable of inducing differentiation of ganglion cells if expressed in cells restricted to amacrine and photoreceptor cell fates. However, Atoh7 was not sufficient to alter differentiation significantly if expressed in cells committed to the photoreceptor lineage (Prasov and Glaser, 2012a; Mao et al., 2013). Together these findings suggest a differential susceptibility of RPCs and differentiating RPCs towards Atoh7 function.
Several upstream and downstream targets of Atoh7 have been identified (Del Bene et al., 2007; Souren et al., 2009). Notably it was shown that Atoh7 is capable of activating its own promoter and thus inducing its own expression in a positive feed forward loop (Del Bene et al., 2007; Skowronska-Krawczyk, 2004; Matter-Sadzinski et al., 2001). Furthermore it was observed that Atoh7 can activate different targets in a dose dependent manner (Del Bene et al., 2007).
In this study we have addressed the differential responsiveness of RPCs to precocious atoh7 expression in vivo, using medaka (Oryzias latipes). We used an rx2 promoter to drive panocular atoh7 expression, starting already in RPCs of the optic vesicle and lasting until the onset of retinal differentiation. We observed a differential response of distinct retinal domains to the precocious atoh7 expression. The dorsal retina developed largely normally. Here, only a significant reduction of cells within the INL (Müller glia cells) was observed. However, the differentiation of the ventral retina, specifically the optic fissure region, was disturbed severely. Here, the photoreceptors layer was absent and the number of Müller glia cells was severely reduced while ganglion cells represented the majority of cells. The abnormal development and differentiation of this ventral retinal domain affected the closure of the optic fissure, resulting in coloboma.
2. Results
2.1. Homogenous precocious atoh7 expression in early RPCs
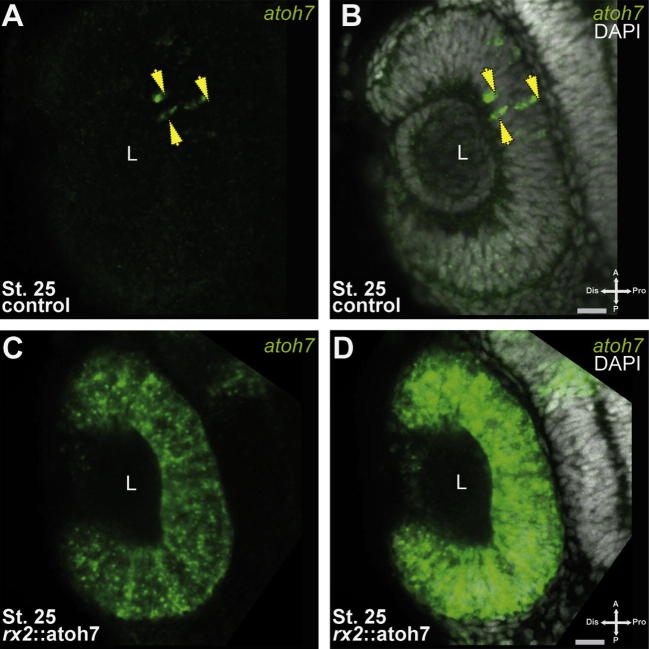
To investigate the putative differential response of multipotent RPCs to atoh7 expression, we aimed to establish precocious atoh7 expression during early eye development. To this end we produced a construct containing a regulatory region of the retinal homeobox gene two (rx2) (Souren et al., 2009; Martinez-Morales et al., 2009) driving atoh7 expression and generated a transgenic medaka line. The rx2 promoter in medaka is active during early eye development from the optic vesicle stage until the onset of differentiation in all RPCs (Martinez-Morales et al., 2009). We confirmed the precocious atoh7 expression by fluorescent in situ hybridization. In rx2::atoh7 embryos, atoh7 mRNA was detectable already at stage 22 (Supplemental Fig. 1) showing abundant expression in the whole developing optic cup. In controls, however, the first atoh7 expressing cells were detected at stage 25 (Fig. 1A, B) in the central retina. At this stage rx2::atoh7 embryos showed a persistent strong and homogenous expression in the optic cup (Fig. 1C, D). Thus our data show that expression of atoh7 directed by an rx2 promoter resulted in precocious homogeneous expression in the forming optic cup already at stage 22, three developmental stages prior to the expression of the endogenous atoh7, which normally marks the onset of differentiation (Del Bene et al., 2007; Kitambi and Malicki, 2008).
Fig. 1.
Homogenous atoh7 expression in early RPCs. (A–D) Whole mount fluorescent in situ hybridization, green: atoh7 mRNA, grey: DAPI nuclear labeling (A–B) wild type, (C–D) rx2:atoh7; scale bar 20 μm. Note the early homogeneous atoh7 expression in rx2::atoh7 embryos, compared to the atoh7 expressing cells restricted to the central optic cup of controls (arrows in A–B).
2.2. Differential responses to precocious homogenous atoh7 expression
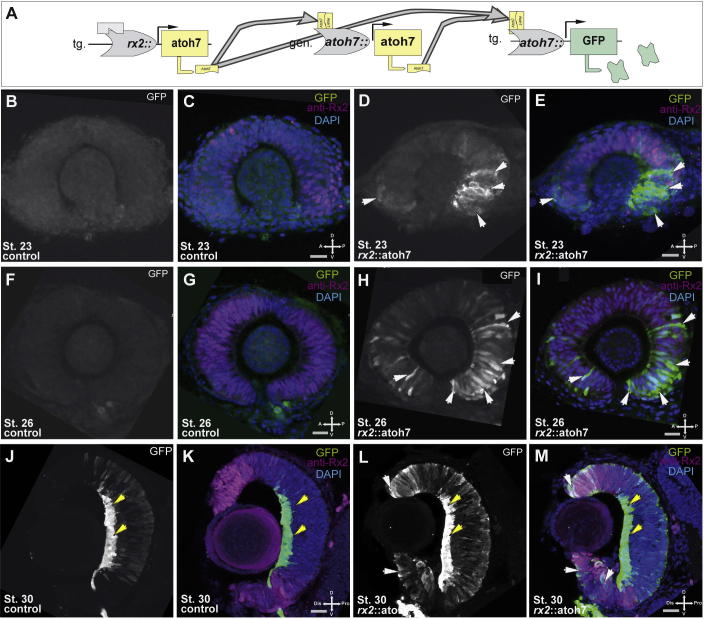
Next we asked if the precocious atoh7 expression in RPCs is functional and results in target gene activation. Atoh7 has been shown to activate its own expression (Del Bene et al., 2007; Skowronska-Krawczyk, 2004; Matter-Sadzinski et al., 2001). Therefore we used an atoh7::GFP reporter line to address functionality of Atoh7 (Fig. 2A) (Del Bene et al., 2007). While in atoh7::GFP embryos the first GFP positive cells in the eye become visible at stage 25/26 (Supplemental Fig. 2), GFP positive cells were visible already at stage 22/23 in rx2::atoh7/atoh7::GFP embryos. Notably the GFP positive cells were heterogeneously distributed over the developing optic cup and mainly detected in the ventral posterior region (Fig. 2D, E, Fig. 2H, I, see F, G as control). At stage 30, during retinal differentiation, GFP positive cells can be found predominantly in the developing ganglion cell layer in the central retina of controls (Fig. 2J, K). However, in rx2::atoh7/atoh7::GFP embryos, additional GFP positive cells were observed in distal retinal regions (Fig. 2L, M).
Fig. 2.
Differential response to precocious homogenous atoh7 expression. (A) Schematic representation of the construct where precocious atoh7 is driven by an rx2 promoter, potentially resulting in a positive feed forward loop, activating the genomic atoh7 promoter and an atoh7::GFP reporter construct. (B–M) Immunohistochemistry for rx2 protein (magenta) on atoh7::GFP reporter embryos, controls (B, C, F, G, J, and K) rx2::atoh7 (D, E, H, I, L and M), at different developmental stages, stage 23, lateral view (B–E) stage 26 lateral view (F–I) and stage 30 (frontal view), DAPI nuclear labeling, scale bar 20 μm. Note the precocious reporter activation in rx2::atoh7 embryos predominantly in ventral regions of the optic cup (D, E, H and I, see B, C, F and G as control). Notably at stage 30 atoh7 reporter activity can mainly be observed in the developing GCL in controls (J, K) whereas a broader reporter activity can be seen in rx2::atoh7 embryos including the apical central retinal domains and the distal retinal domains.
Together our data indicate that precocious expression of atoh7 results in precocious target gene activation (atoh7::GFP) and therefore is functional. However, the susceptibility of RPCs within the forming optic cup to respond to the precocious atoh7 expression was found to be diverse. The strongest response was detected in the ventral/posterior optic cup.
2.3. Precocious atoh7 expression results in Coloboma
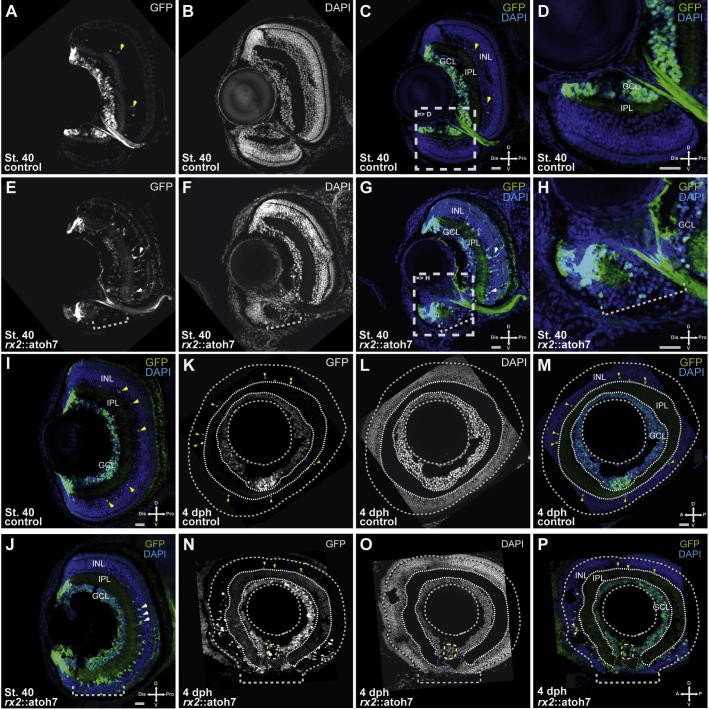
To study the consequence of the precocious atoh7 expression for retinal development we investigated retinal layering and morphology of postembryonic hatchlings. Surprisingly the dorsal retina of rx2::atoh7/atoh7::GFP hatchlings was found to have differentiated largely normally with correct layering (Fig. 3E–H, see A–D as control). However, while GFP positive cells driven by the atoh7 promoter, were detected mainly in the GCL and sparsely in the INL of controls (Fig. 3A–D) more GFP positive cells were observed in the INL of rx2::atoh7/atoh7::GFP fish, reflecting the rx2 driven atoh7 expression in differentiated Müller glia cells. Furthermore additional expression was found in the ONL (Fig. 3E–H).
Fig. 3.
Coloboma as a result of precocious atoh7 expression in RPCs. (A–P) Cryosections of atoh7::GFP (controls) (A–D, I, K–M) and rx2::atoh7/atoh7::GFP (E–H, J, N–P) hatchlings, frontal (A–J) and lateral (K–P). Scale bar 20 μm; GCL = Ganglion cell layer; IPL = inner plexiform layer; INL = Inner nuclear layer, dph = days post hatch. While retinal patterning into GCL, INL and ONL can be observed in controls (B–D, I), retinal layering is largely disturbed in the ventral retina of rx2::atoh7 hatchlings (F–H, J). Atoh7 driven GFP expression can be observed in the GCL and amacrine cells of the INL (A, yellow arrowheads) of controls, whereas GFP expression is extended to additional cells of the INL (Müller glia cells) and can also be observed in the malformed ventral retinal domain (E–H) of rx2::atoh7 hatchlings. Lateral sections of rx2::atoh7 fish indicate that the largely affected ventral domain equates with the optic fissure region (N–P), see K–M as control. Notably the optic fissure is not orderly fused in rx2::atoh7 fish.
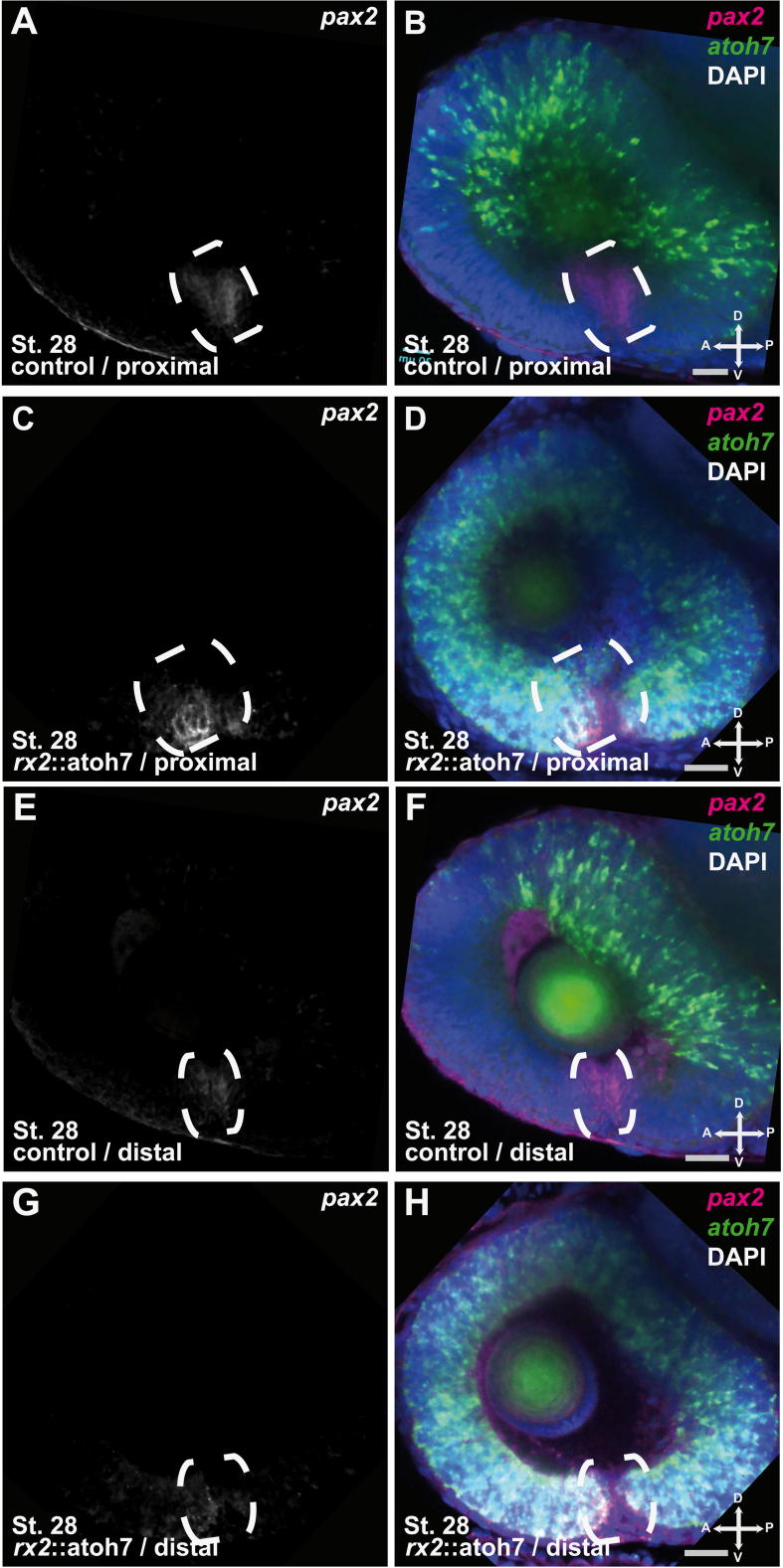
In contrast, the ventral retina was affected severely. The retinal layering in the rx2::atoh7 hatchlings was found to be disrupted by fused GCL and INL and the ONL was completely absent (Fig. 3G, H, see C, D as control). This phenotype was most pronounced close to the optic nerve head (Fig. 3J, see I as control), the most proximal region of the optic fissure, but also found along the optic fissure margins. Notably an optic nerve head is also established in rx2::atoh7 fish (Supplemental Fig. 4) although showing an slightly altered morphology. To address whether a misspecification of the ventral optic cup, including the optic fissure margins is causing this phenotype in rx2::atoh7 embryos we analyzed the expression of a ventral optic cup marker (pax2). Its unaltered expression in rx2::atoh7 embryos clearly indicates a normal specification of the ventral optic cup (Supplemental Fig. 5).
In a lateral view the persisting optic fissure of the rx2::atoh7/atoh7::GFP hatchlings can be clearly visualized (Fig. 3N–P, see K–M as control). Taken together this indicates that distinct retinal domains respond differentially to precocious atoh7 expression. The dorsal retina is only affected to a minor extent. The former optic fissure region of the ventral retina on the other hand was affected severely, resulting in coloboma.
2.4. Precocious atoh7 expression results in changes of retinal cell numbers
As described above the dorsal retinal domain appeared to be largely normal with correct layering. To further investigate a potentially subtle phenotype with respect to retinal differentiation we quantified cells in the distinct layers of the dorsal retina (GCL: ganglion cells, INL: amacrine cells, horizontal cells, bipolar cells and Müller glia cells and ONL: photoreceptor cells) of rx2::atoh7 hatchlings and controls (Fig. 4).
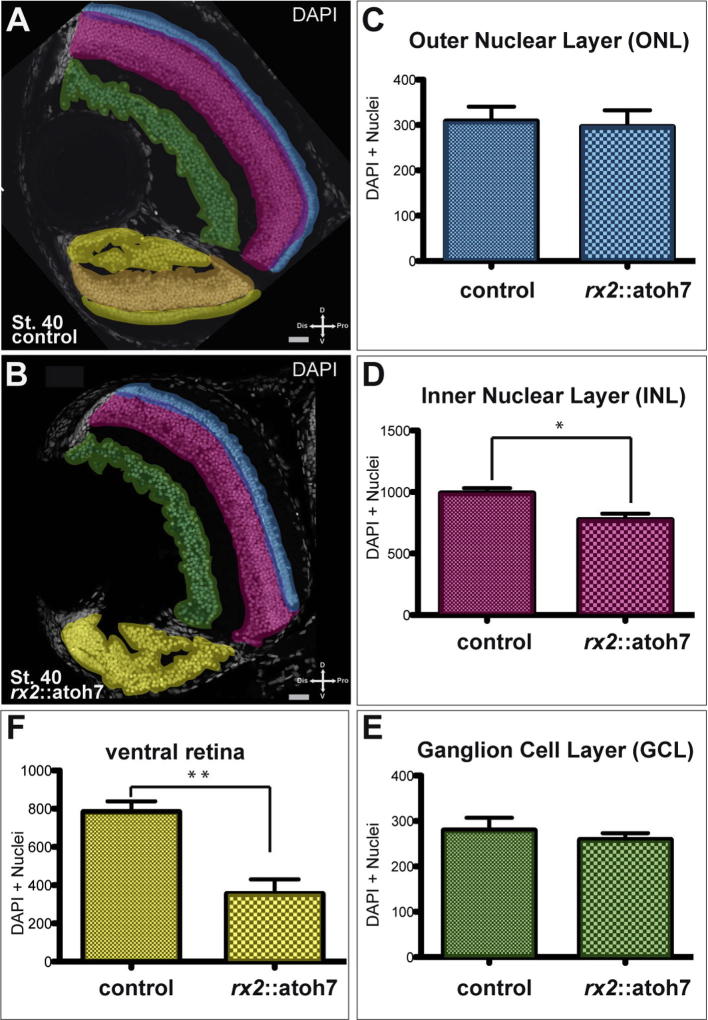
Fig. 4.
Quantification of cell numbers in rx2::atoh7 hatchlings. (A, B) Frontal sections of hatchling eyes, controls (A, n = 6; 6 fish) and rx2:atoh7 (n = 7; 4 fish) were used for quantification of DAPI stained nuclei within different retinal domains. Graphs are presented as mean and SEM. In the dorsal retina, GCL (green, E), INL (magenta, D) and ONL (blue, C) were analyzed. A significantly reduced number of nuclei of the INL (D) was found in rx2::atoh7 fish. In the ventral retina a separation into GCL, INL and ONL was impossible in rx2::atoh7 fish. Therefore the ventral domain was quantified in toto. A significant reduction of nuclei was found in this domain in the rx2::atoh7 fish (F).
Nuclear quantification of the dorsal GCL of rx2::atoh7 hatchlings did not show an increase in number (Fig. 4 A, B, E, p value = 0.5826). In addition the ONL was not significantly changed (Fig. 4 A, B, C, p value = 0.5608). However, significantly fewer cells were found in the INL of rx2::atoh7 hatchlings (Fig. 4 A, B, D, p value = 0.0130). In the malformed ventral retina of rx2::atoh7 discrimination of the distinct layers was impossible. Therefore the quantification was performed for the indistinct and merged ventral retina and compared to the composite ventral layers of the controls (Fig. 4A, B). Quantification of nuclei revealed a significant reduction in rx2::atoh7 hatchlings (Fig. 4A, B, F, p value = 0.0019). In summary nuclear quantification revealed a significant reduction of the dorsal INL, showing that also the dorsal retina of rx2::atoh7 hatchlings is affected by precocious expression. Furthermore the malformed ventral retina showed a significantly reduced number of cells.
2.5. RPCs of the ventral retina of rx2::atoh7 hatchlings differentiated predominantly into RGCs
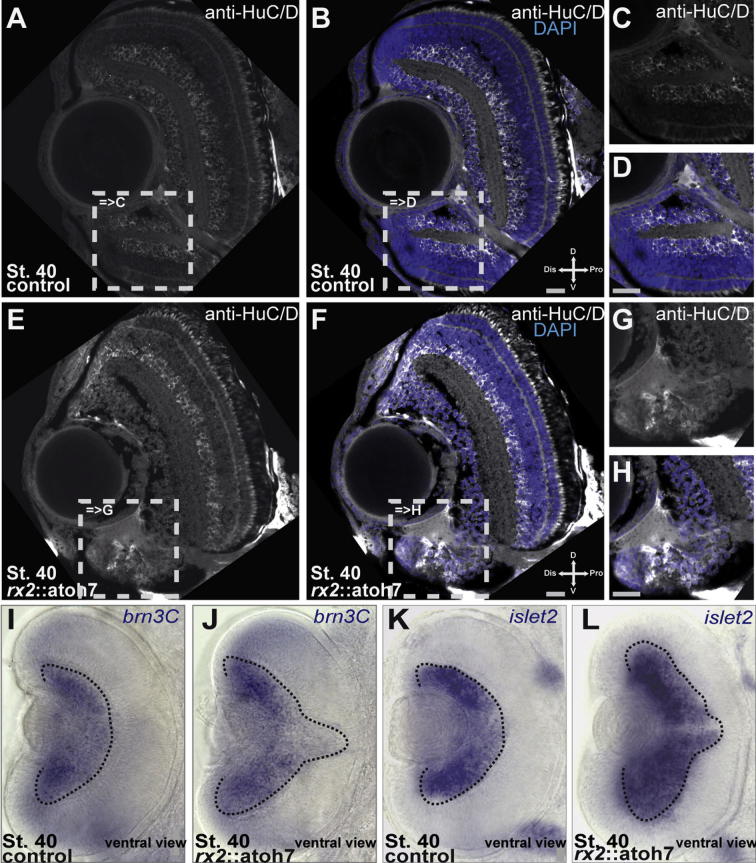
To further investigate the ventral phenotype of rx2::atoh7 eyes, we performed immunohistochemistry (a-HuC/D) and in situ hybridization (brn3c, islet2) for ganglion cell markers. In control retinae, HuC/D positive cells are located in the GCL and in the basal part of the inner INL (Fig. 5A–D). In contrast the majority of cells in the ventral retina of rx2::atoh7 hatchlings are positive for HuC/D, indicating an enhanced differentiation into ganglion or amacrine cells. In control retinae brn3C and islet2 mRNAs were detectable only in the GCL (Fig. 5I, K). The expression of brn3c and islet2 in rx2::atoh7 hatchlings, however, was also found beyond the normal GCL, along the optic fissure (Fig. 5J, L). Taken together our results strongly indicate a predominant differentiation into ganglion cells in the ventral retina, specifically along the former optic fissure margins.
Fig. 5.
Precocious atoh7 expression results in predominant RGC differentiation in the ventral retina. (A–H) Anti-HuC/D immunohistochemistry performed on frontal sections of control (A–D) and rx2::atoh7 (E–H) hatchlings, scale bar 20 μm. Notably a broader HuC/D positive area can be observed in the ventral retina of rx2::atoh7 fish. (I–L) ventral views of whole mount in situ hybridizations performed with probes for brn3c (I, control and J, rx2::atoh7) and islet2 (K, control and L, rx2::atoh7). Notably an extended expression of brn3c and islet2 along the optic fissure region can be observed in rx2::atoh7 hatchlings.
2.6. Reduction of late born cell types in rx2::atoh7 hatchlings
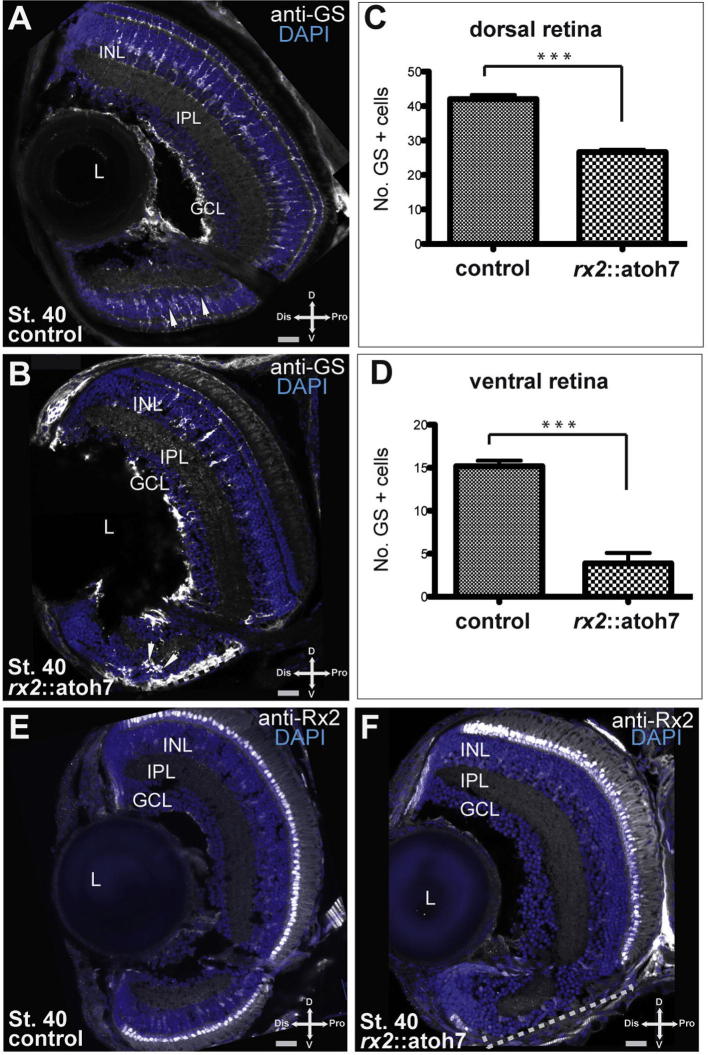
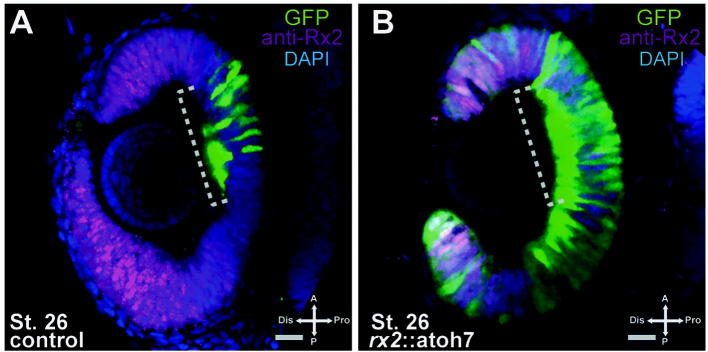
Our data indicate that precocious atoh7 expression results in a reduction of cells in the dorsal INL. We hypothesized that the cause for this reduction could be a loss of late born cell types (e.g. Müller glia cells) as reported for clonal fate shifts of atoh7 (Kanekar et al., 1997). To test this hypothesis, we quantified Müller glia cells based on immunohistochemistry for glutamine synthetase (GS). Quantification of the GS stained hatchling retinae revealed a significant decrease of Müller glia cells in rx2::atoh7 hatchlings, compared to controls (Fig. 6A–H,) in dorsal (p value <0.0001) and ventral (p value <0.0001) retinal domains. In addition to the reduction of Müller glia cells we observed an absence of nuclei in the ONL of a ventral retinal domain of rx2::atoh7 hatchlings (see above) presumably the result of missing photoreceptor cells. To further demonstrate the loss of photoreceptor cells, we performed Rx2 immunohistochemistry, as Rx2 is expressed in all photoreceptor cells. In rx2::atoh7 hatchlings a ventral retinal area in which photoreceptor cells are absent was detected (Fig. 6J, L, see I, K as control). Therefore we conclude that the precocious atoh7 expression in all RPCs results in a reduction of late born cell types. Notably, in the dorsal retina this reduction does not result in an increase of RGCs. However, in the ventral retina, specifically in the former optic fissure region, the loss of late born cell types is accompanied by a predominant differentiation into RGCs.
Fig. 6.
Reduction of late born retinal cell types as a result of precocious atoh7 expression. (A and B) Immunohistochemistry performed with anti-GS (Müller glia cells) on frontal sections of hatchling eyes of control (A) and rx2::atoh7 (B); scale bar 20 μm, GCL = Ganglion cell layer; IPL = Inner plexiform Layer; INL = Inner nuclear layer; L = lens. GS-positive Müller glia cells (arrowheads) were quantified in the dorsal and ventral retina respectively (C and D), mean and SEM values are plotted. In both domains a significant reduction in GS-positive Müller glia cells was found. (E and F) Immunohistochemistry performed with anti-Rx2, indicating a loss of photoreceptor cells in the ONL of rx2::atoh7 fish (F), see E as control.
3. Discussion
In this study we have investigated the differential response of RPCs to precocious and panocular expression of atoh7. We found that, although expressed in the entire optic vesicle/optic cup, Atoh7 was early on functional only in distinct retinal domains (Fig. 7 Scheme). The functionality was assessed by an atoh7::GFP line exploiting the fact that Atoh7 is activating its own expression (Del Bene et al., 2007; Skowronska-Krawczyk, 2004; Matter-Sadzinski et al., 2001). By this approach we clearly identified regions within the retina, which are responsive to precious atoh7 expression. In contrast we show also that expression of atoh7 is no guarantee of its functionality. This important prerequisite could not be addressed in previous studies (Prasov and Glaser, 2012b; Mao et al., 2013; Kanekar et al., 1997; Wang and Harris, 2005).
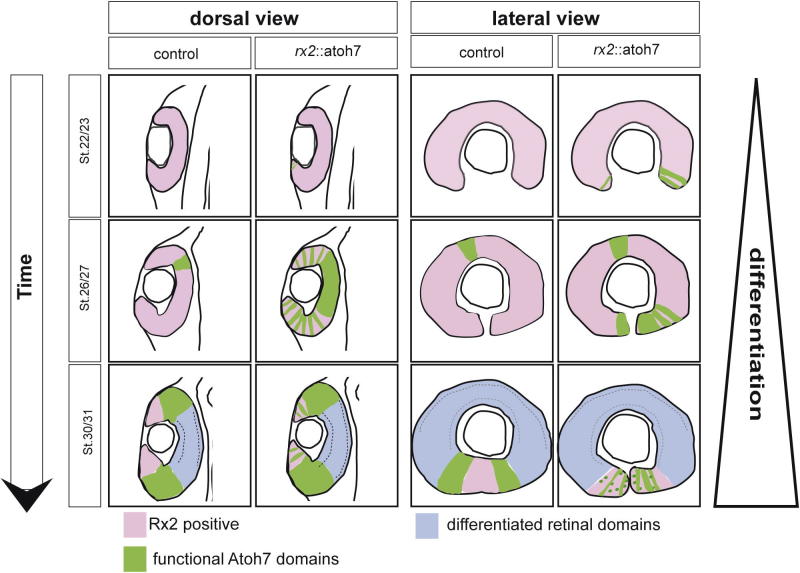
Fig. 7.
Schematic summary of differential Atoh7 function Dorsal and lateral schematic views of the eyes from controls and rx2::atoh7 embryos at different developmental stages. The schemes are based on the atoh7::GFP activation and Rx2 immunohistochemistry. The lateral view shows a central plane in terms of the proximal/distal axis. The dorsal view shows a central plane in terms of the dorsal/ventral axis.
Our findings in the dorsal retina of rx2::atoh7 fish show a significant reduction in late born cell types (Müller glia cells) in response to a depletion of the pool of RPCs by the precocious differentiation of RPCs into RGCs. Based on data derived from clonal analysis, this precocious differentiation did not result in an increased total number of dorsal RGCs as might have been predicted (Kanekar et al., 1997; Wang and Harris, 2005). Importantly the number of RGCs is likely to be stringently regulated (He et al., 2012; Livesey and Cepko, 2001; Bassett and Wallace, 2012; Chiodini et al., 2013). This, however, cannot be assessed if analyzed clonally (Kanekar et al., 1997; Wang and Harris, 2005). This tight regulation is overcome, however, if for example, Atoh7 activity is maintained in subsequent lineages as shown here and in mice where atoh7 was introduced into the NeuroD locus (Mao et al., 2013).
The phenotype we observed in the ventral retina of rx2::atoh7 fish was more profound, specifically in the margins of the optic fissure. In addition to a reduction of Müller glia cells, the ONL consisting of late born photoreceptors was absent. Furthermore, the INL and GCL were found to be fused. Notably, the onset of Atoh7 function in the ventral retina, especially in the posterior fissure margin, preceded the physiological onset by several developmental stages (Fig. 7 Scheme), however, not altering cell identity in the ventral optic cup (Supplemental Fig. 4). This precocious Atoh7 functionality can be explained by the coexpression of six3 (Loosli et al., 1998) a cofactor of Atoh7 (Tessmar et al., 2002; Wang and Harris, 2005) in the ectopic ventral domain. As in Xenopus (Wang and Harris, 2005) this may result in a complete switch of RPCs towards RGC fate.
While during normal development the optic fissure represents a transient gap and is fused as development proceeds, a remaining fissure is persistent in rx2::atoh7 fish (Fig. 7 Scheme). This so-called “coloboma”, can have various origins (Gregory-Evans, 2004; Gregory-Evans, 2013). We propose that the reason for coloboma in rx2::atoh7 fish is the precocious differentiation of the optic fissure margins (predominantly into RGCs) and not simply misspecification of the ventral optic cup.
Notably a coloboma was also observed in Hes1 Knock Out mice (Lee et al., 2005). Hes1 is a repressor of Atoh7 (Matter-Sadzinski, 2005; Souren et al., 2009; Lee et al., 2005) and loss of Hes1 resulted in a precocious expression of atoh7 (Lee et al., 2005) also without affecting ventral retinal identity. Considering our data, it is conceivable that such precocious atoh7 expression in Hes1 Knock Out mice, could also result in precocious retinal differentiation finally resulting in coloboma.
4. Materials Methods
4.1. Animals and transgenic lines
Medaka were maintained in the fish facility of COS Uni-Heidelberg (in accordance to Tierschutzgesetz 111, Abs. 1, Nr. 1 and with European Union animal welfare guidelines). Wild-type Cab Oryzias latipes were kept as a closed stock and staged according to Iwamatsu (Iwamatsu, 2004). atoh7::GFP line is formerly known and published as ath5::GFP line (Del Bene et al., 2007). Sc(rx2::atoh7 cmlc2::eGFP) transgenic fish were created by microinjection with Meganuclease as previously described (Thermes et al., 2003).
4.2. Cloning
The O. latipes atoh7 cDNA was cloned into a Gateway middle entry vector and a regulatory region of rx2 (Souren et al., 2009; Martinez-Morales et al., 2009) was used in a 5′entry vector. As 3′entry a standard Sv40PolyA was used. The Sc(rx2::atoh7 cmlc::GFP) construct was assembled in a Gateway destination vector containing IsceI transgenesis sites and an cmlc2::eGFP screening cassette via three-way-gateway reaction according to manufacturers protocol (Invitrogen).
4.3. Whole mount in situ hybridization
In situ probes were generated as previously described (Del Bene et al., 2007; Souren et al., 2009; Loosli et al., 2001). Whole-mount in situ hybridization for medaka brn3C and islet2 was carried out as previously described (Loosli et al., 2001). Samples were mounted in 87% Glycerol and imaged with a Leica DM5000B, 10× or 20× air objectives, Leica CD500 microscope camera and Leica FireCam 1.7.1 software. Whole-mount in situ hybridization for medaka atoh7 was performed with a TSA KIT (Perkin Elmer) (Souren et al., 2009). The Atoh7 in situ probe was labeled with Digoxygenin and developed with FITC TSA for 1 h in the dark. Samples were mounted in 1.5% low melting agarose. Confocal stacks were taken using the Leica SP5 confocal microscope, 20× water immersion objective and Leica Application Suite (LAS) software. Two color whole-mount in situ hybridization for pax2 (fast red, Roche) and atoh7 (TSA) was performed as described previously in Souren et al., 2009.
4.4. Immunohistochemistry
For immunohistochemistry embryos were fixed at the indicated stages in 4% PFA in 1 x PTW over night at 4 °C. Fixed embryos were either heated (Inoue and Wittbrodt, 2011) and mounted for cryo-sectioning or stained whole mount according to the protocols adapted from the zebrafish book (Westerfield, 2000). In case of sectioning, frontal or lateral cryosections (as indicated) of 16 μm thickness were prepared and stained as described in (Inoue and Wittbrodt, 2011). The following primary antibodies were used: anti-GS (mouse, Chemicon MAB302, 1:500), anti-Rx2 (rabbit, self-made, 1:250, anti-olRx2 antibody was raised against the full-length olRx2 (NP_001098373.1) recombinant protein in rabbits (Charles River), and affinity purified using the antigen coupled to AffiGel (Biorad) (Herder et al., 2013), anti-GFP (chicken, life technologies A10262, 1:500) and for anti-HuCD (mouse, invitrogen A-21271, 1:250) and anti-acetylated Tubulin (mouse, SIGMA T7451-100UL, 1:100). The following secondary antibodies were used at a 1:500 dilution, anti-mouse Cy5 (Jackson 715-175-151); anti mouse 546 (invitrogen A-11030), anti chicken 488 (Jackson 703-545-155), anti rabbit 549 (Jackson 112-505-144). DAPI (SIGMA, D956) nuclear counterstaining was performed (Inoue and Wittbrodt, 2011).
4.5. Imaging and image processing
All images were acquired by confocal microscopy (Leica TCS SPE and Leica SP5). Images were acquired with either 20× water objective or 40× oil objective. Shown are either single planes of a z-Stack or max projections of a few micrometers (0.5 μm to 3 μm). Images were processed via Fiji image processing software to adjust brightness and contrast, stitched (http://fly.mpi-cbg.de/~preibisch/) if necessary and followed by application of the pure de-noise plugin on the final picture with standard automated settings and 6 cycles of de-noising (http://bigwww.epfl.ch/algorithms/denoise/).
4.6. Cell counting procedure
A MatLab based processing algorithm was programmed to segment the acquired images for nuclei and to enable consecutive counting. The program allowed the definition of a region of interest, e.g. ONL, INL, GCL. The first step in the process was to compute the average projection of the z-stack that was acquired. This was followed by adaptive histogram equalization as a pre-processing step. A MatLab Image Processing toolbox function called the extended-maxima transform (Soille, 1999) (the regional maxima of the H-maxima transform) was then applied to the equalized image to segment the cells. Finally, the perimeters and centroids of the individual cells were computed to extract the cell count in the different layers. A 0.75 μm thick max-projection of frontal cryosections at optic nerve height was used; control fish (n = 6, 6 eyes) and from an in cross of the rx2::Atohh7 (n = 4; 7 eyes). Müller glia cell (anti-GS) counting was performed by FIJI (http://rsb.info.nih.gov/ij/plugins/cell-counter.html). The optic nerve in frontal sections was used to define ventral and dorsal retinal domains.
5. Author contributions
J.W., R.S. and S.H. designed the experiments. R.S. performed the experiments, R.P. developed the MATLAB program for nuclear counting. R.S., S.H. and J.W. wrote the manuscript.
Acknowledgements
We thank the Wittbrodt department, especially D. Inoue, L. Centanin, L. Poggi, S. Albadri, S. Kirchmaier, B. Höckendorf, R. Reinhard for discussions and S. Kirchmaier for materials. We thank D. Inoue and O. Gruss for the Rx2 antibody. We are grateful to A. Saraceno, E. Leist and M. Majewski for excellent fish husbandry. And we thank F. Gruhl for comments on the manuscript and F. Loosli for discussions. Special thanks to our native english experts N. Foulkes and D. Ibberson for helping with the manuscript.
R.S. received a fellowship LGFG (Funding program of the State of Baden-Württemberg). This work was supported by the Deutsche Forschungsgemeinschaft (DFG) (J.W.) and ERC (J.W.).
Appendix A. Supplementary data
Supplementary Fig. 1.
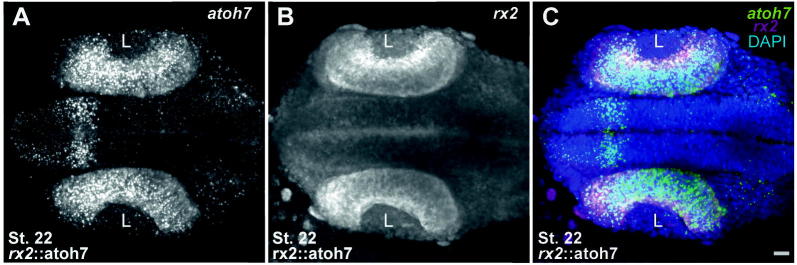
In rx2::atoh7 embryos atoh7 is expressed already at stage 22. (A–C) Double fluorescent in situ hybridization showing atoh7 expressed in an rx2::atoh7 embryo at stage 22 (A: atoh7, B: rx2, C: merge), scalebar = 20 μm.
Supplementary Fig. 2.
Physiological onset of atoh7 expression in the central retina. (A and B) Detected with the atoh7::GFP reporter; atoh7 driven GFP (green), anti-rx2 immunohistochemistry (magenta) with DAPI nuclear counter-labeling (blue), scalebar = 20 μm, note the central onset of atoh7 driven GFP in controls at stage 26. In the periphery no GFP is detectable.
Supplementary Fig. 3.
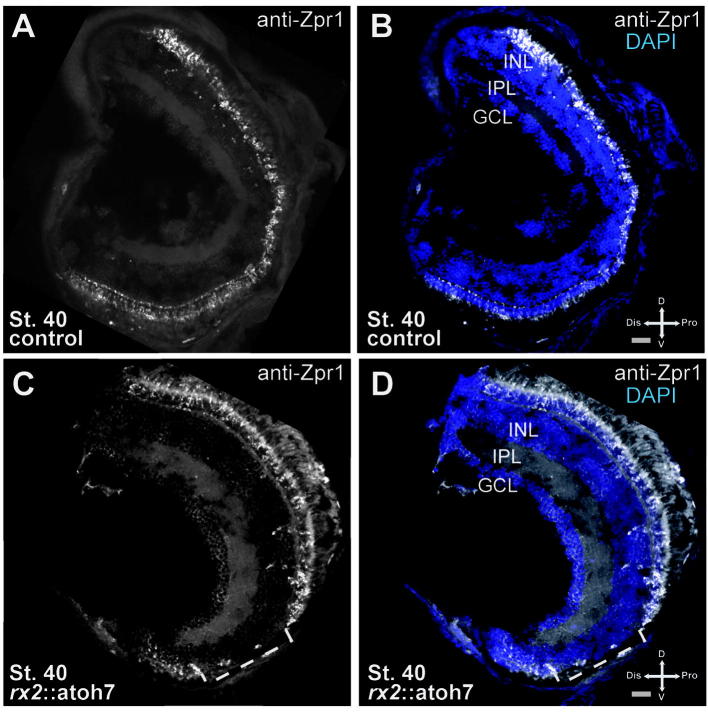
Lack of photoreceptors in the ventral domain of rx2::atoh7 hatchlings. Double cone marker Zpr1 stain in control and rx2::atoh7 in frontal non optic nerve containing sections. (A,B) In controls, the Zpr1 staining is visible along the circumference of the INL. (C,D) In rx2::atoh7 fish a considerable area does not exhibit an ONL and almost no Zpr1 stain could be detected in this area.
Supplementary Fig. 4.
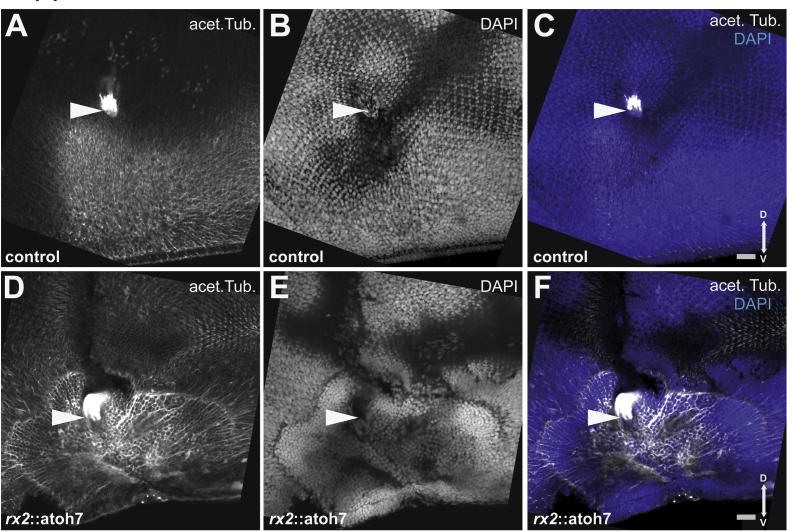
Altered optic nerve head morphology in rx2::atoh7 hatchlings. (A–F) flat mounted retinae, 5 μm maximum projections, viewed from the RPE, acetylated tubulin (acet. Tub., grey), to visualize neuronal processes, nuclear stain (DAPI, blue); scale bar 20 μm. (A–C) regular optic nerve head formation (arrowhead) with differentiated photoreceptors (D–F) altered optic nerve head morphology (arrowhead) and defects in ventral retinal differentiation.
Supplementary Fig. 5.
Unaltered ventral marker expression in rx2::atoh7 embryos. (A–H) Whole mount double fluorescent (TSA, FastRed) in situ hybridizations for pax2 mRNA magenta (fast Red) and atoh7 mRNA green (TSA); DAPI nuclear stain blue; 1 μm optical section, stage 28 laterally mounted, pax2 expression domain encircled, scale bar 20 μm. (A and B) proximal optic cup of control embryo (C and D) proximal optic cup of rx2::atoh7 (E and F) distal optic cup of control embryo (G and H) distal optic cup of rx2::atoh7, note the unaltered pax2 expression domain in the ventral optic cup of rx2::atoh7 embryos.
References
- Bassett E.A., Wallace V.A. Cell fate determination in the vertebrate retina. Trends Neurosci. 2012:1–9. doi: 10.1016/j.tins.2012.05.004. [DOI] [PubMed] [Google Scholar]
- Cepko C.L., Austin C.P., Yang X., Alexiades M., Ezzeddine D. Cell fate determination in the vertebrate retina. Proc. Natl. Acad. Sci. USA. 1996;93:589–595. doi: 10.1073/pnas.93.2.589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiodini F., Matter-Sadzinski L., Rodrigues T., Skowronska-Krawczyk D., Brodier L., Schaad O., Bauer C., Ballivet M., Matter J.-M. A Positive feedback loop between ATOH7 and a notch effector regulates cell-cycle progression and neurogenesis in the retina. Cell Rep. 2013;3:796–807. doi: 10.1016/j.celrep.2013.01.035. [DOI] [PubMed] [Google Scholar]
- Del Bene F., Ettwiller L., Skowronska-Krawczyk D., Baier H., Matter J.-M., Birney E., Wittbrodt J. In vivo validation of a computationally predicted conserved Ath5 target gene set. PLoS Genet. 2007;3:e159. doi: 10.1371/journal.pgen.0030159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuhrmann S. Elsevier; 2010. Current Topics in Developmental Biology. [Google Scholar]
- Gonzalez-Hoyuela M., Barbas J.A., Rodriguez-Tebar A. The autoregulation of retinal ganglion cell number. Development. 2001;128:117–124. doi: 10.1242/dev.128.1.117. [DOI] [PubMed] [Google Scholar]
- Gregory-Evans C.Y. Ocular coloboma: a reassessment in the age of molecular neuroscience. J. Med. Genet. 2004;41:881–891. doi: 10.1136/jmg.2004.025494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gregory-Evans C.Y., Wallace V.A., Gregory-Evans K. Gene networks: dissecting pathways in retinal development and disease. Prog. Retin. Eye Res. 2013;33:40–66. doi: 10.1016/j.preteyeres.2012.10.003. [DOI] [PubMed] [Google Scholar]
- He J., Zhang G., Almeida A.D., Cayouette M., Simons B.D., Harris W.A. How variable clones build an invariant retina. Neuron. 2012;75:786–798. doi: 10.1016/j.neuron.2012.06.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herder C., Swiercz J.M., Muller C., Peravali R., Quiring R., Offermanns S., Wittbrodt J., Loosli F. ArhGEF18 regulates RhoA-Rock2 signaling to maintain neuro-epithelial apico-basal polarity and proliferation. Development. 2013;140:2787–2797. doi: 10.1242/dev.096487. [DOI] [PubMed] [Google Scholar]
- Inoue D., Wittbrodt J. One for all—A highly efficient and versatile method for fluorescent immunostaining in fish embryos. PLoS ONE. 2011;6:e19713. doi: 10.1371/journal.pone.0019713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iwamatsu T. Stages of normal development in the medaka Oryzias latipes. Mech. Dev. 2004;121:605–618. doi: 10.1016/j.mod.2004.03.012. [DOI] [PubMed] [Google Scholar]
- Kanekar S., Perron M., Dorsky R., Harris W.A., Jan L.Y., Jan Y.N., Vetter M.L. Xath5 participates in a network of bHLH genes in the developing Xenopus retina. Neuron. 1997;19:981–994. doi: 10.1016/s0896-6273(00)80391-8. [DOI] [PubMed] [Google Scholar]
- Kay J.N. Staggered cell-intrinsic timing of ath5 expression underlies the wave of ganglion cell neurogenesis in the zebrafish retina. Development. 2005;132:2573–2585. doi: 10.1242/dev.01831. [DOI] [PubMed] [Google Scholar]
- Kay J.N., Finger-Baier K.C., Roeser T., Staub W., Baier H. Retinal ganglion cell genesis requires lakritz, a zebrafish atonal homolog. Neuron. 2001;30:725–736. doi: 10.1016/s0896-6273(01)00312-9. [DOI] [PubMed] [Google Scholar]
- Kitambi S.S., Malicki J.J. Spatiotemporal features of neurogenesis in the retina of medaka, Oryzias latipes. Dev. Dyn. 2008;237:3870–3881. doi: 10.1002/dvdy.21797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le T.T., Wroblewski E., Patel S., Riesenberg A.N., Brown N.L. Math5 is required for both early retinal neuron differentiation and cell cycle progression. Dev. Biol. 2006;295:764–778. doi: 10.1016/j.ydbio.2006.03.055. [DOI] [PubMed] [Google Scholar]
- Lee H., Wroblewski E., Philips G., Stair C., Conley K., Reedy M., Mastick G., Brown N. Multiple requirements for Hes1 during early eye formation. Dev. Biol. 2005;284:464–478. doi: 10.1016/j.ydbio.2005.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Z., Joseph N.M., Easter S.S. The morphogenesis of the zebrafish eye, including a fate map of the optic vesicle. Dev. Dyn. 2000;218:175–188. doi: 10.1002/(SICI)1097-0177(200005)218:1<175::AID-DVDY15>3.0.CO;2-K. [DOI] [PubMed] [Google Scholar]
- Livesey F.J., Cepko C.L. Vertebrate neural cell-fate determination: lessons from the retina. Nat. Rev. Neurosci. 2001;2:109–118. doi: 10.1038/35053522. [DOI] [PubMed] [Google Scholar]
- Loosli F.F., Köster R.W.R., Carl M.M., Krone A.A., Wittbrodt J.J. Six3, a medaka homologue of the Drosophila homeobox gene sine oculis is expressed in the anterior embryonic shield and the developing eye. Mech. Dev. 1998;74:159–164. doi: 10.1016/s0925-4773(98)00055-0. [DOI] [PubMed] [Google Scholar]
- Loosli F., Winkler S., Burgtorf C., Wurmbach E., Ansorge W., Henrich T., Grabher C., Arendt D., Carl M., Krone A. Medaka eyeless is the key factor linking retinal determination and eye growth. Development. 2001;128:4035–4044. doi: 10.1242/dev.128.20.4035. [DOI] [PubMed] [Google Scholar]
- Malicki J. Cell fate decisions and patterning in the vertebrate retina: the importance of timing, asymmetry, polarity and waves. Curr. Opin. Neurobiol. 2004;14:15–21. doi: 10.1016/j.conb.2004.01.015. [DOI] [PubMed] [Google Scholar]
- Mao C.A., Cho J.H., Wang J., Gao Z., Pan P., Tsai W.W., Frishman L.J., Klein W.H. Reprogramming amacrine and photoreceptor progenitors into retinal ganglion cells by replacing Neurod1 with Atoh7. Development. 2013;140:541–551. doi: 10.1242/dev.085886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinez-Morales J.-R., Wittbrodt J. Shaping the vertebrate eye. Curr. Opin. Genet. Dev. 2009;19:511–517. doi: 10.1016/j.gde.2009.08.003. [DOI] [PubMed] [Google Scholar]
- Martinez-Morales J.-R., Del Bene F., Nica G., Hammerschmidt M., Bovolenta P., Wittbrodt J. Differentiation of the vertebrate retina is coordinated by an FGF signaling center. Dev. Cell. 2005;8:565–574. doi: 10.1016/j.devcel.2005.01.022. [DOI] [PubMed] [Google Scholar]
- Martinez-Morales J.R., Rembold M., Greger K., Simpson J.C., Brown K.E., Quiring R., Pepperkok R., Martin-Bermudo M.D., Himmelbauer H., Wittbrodt J. Ojoplano-mediated basal constriction is essential for optic cup morphogenesis. Development. 2009;136:2165–2175. doi: 10.1242/dev.033563. [DOI] [PubMed] [Google Scholar]
- Masai I., Stemple D.L., Okamoto H., Wilson S.W. Midline signals regulate retinal neurogenesis in zebrafish. Neuron. 2000;27:251–263. doi: 10.1016/s0896-6273(00)00034-9. [DOI] [PubMed] [Google Scholar]
- Matter-Sadzinski L. A bHLH transcriptional network regulating the specification of retinal ganglion cells. Development. 2005;132:3907–3921. doi: 10.1242/dev.01960. [DOI] [PubMed] [Google Scholar]
- Matter-Sadzinski L., Matter J.-M., Ong M.-T., Hernandez J., Ballivet M. Specification of neurotransmitter receptor identity in developing retina: the chick ATH5 promoter integrates the positive and negative effects of several bHLH proteins. Development. 2001;128:217–231. doi: 10.1242/dev.128.2.217. [DOI] [PubMed] [Google Scholar]
- Prasov L., Glaser T. Dynamic expression of ganglion cell markers in retinal progenitors during the terminal cell cycle. Mol. Cell. Neurosci. 2012;50:160–168. doi: 10.1016/j.mcn.2012.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prasov L., Glaser T. Pushing the envelope of retinal ganglion cell genesis Context dependent function of Math5 (Atoh7) Dev. Biol. 2012;368:214–230. doi: 10.1016/j.ydbio.2012.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rembold M. Individual cell migration serves as the driving force for optic vesicle evagination. Science. 2006;313:1130–1134. doi: 10.1126/science.1127144. [DOI] [PubMed] [Google Scholar]
- Run-Tao Yan W.M.L.L.S.-Z.W. BHLH genes and retinal cell fate specification. Mol. Neurobiol. 2005;32 doi: 10.1385/MN:32:2:157. 157-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sinn R., Wittbrodt J. An eye on eye development. Mech. Dev. 2013;130:347–358. doi: 10.1016/j.mod.2013.05.001. [DOI] [PubMed] [Google Scholar]
- Skowronska-Krawczyk D. Highly specific interactions between bHLH transcription factors and chromatin during retina development. Development. 2004;131:4447–4454. doi: 10.1242/dev.01302. [DOI] [PubMed] [Google Scholar]
- Soille P. Springer-Verlag; 1999. Morphological Image Analysis: Principles and Applications; pp. 170–171. [Google Scholar]
- Souren M., Martinez-Morales J., Makri P., Wittbrodt B., Wittbrodt J. A global survey identifies novel upstream components of the Ath5 neurogenic network. Genome Biol. 2009;10:R16–R92. doi: 10.1186/gb-2009-10-9-r92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tessmar K., Loosli F., Wittbrodt J. A screen for co-factors of Six3. Mech. Dev. 2002;117:103–113. doi: 10.1016/s0925-4773(02)00185-5. [DOI] [PubMed] [Google Scholar]
- Thermes V., Grabher C., Ristoratore F., Bourrat F., Choulika A., Wittbrodt J., Joly J.-S. Erratum to: “I-SceI meganuclease mediates highly efficient transgenesis in fish”. Mech. Dev. 2003;120:267. doi: 10.1016/s0925-4773(02)00218-6. [DOI] [PubMed] [Google Scholar]
- Wang J.C.C., Harris W.A. The role of combinational coding by homeodomain and bHLH transcription factors in retinal cell fate specification. Dev. Biol. 2005;285:101–115. doi: 10.1016/j.ydbio.2005.05.041. [DOI] [PubMed] [Google Scholar]
- Westerfield M. fourth ed. Univ. of Oregon Press; Eugene: 2000. The zebrafish book. A guide for the laboratory use of zebrafish (Danio rerio) [Google Scholar]
- Wong L.L., Rapaport D.H. Defining retinal progenitor cell competence in Xenopus laevis by clonal analysis. Development. 2009;136:1707–1715. doi: 10.1242/dev.027607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang X.M., Yang X.J. Regulation of retinal ganglion cell production by Sonic hedgehog. Development. 2001;128:943–957. doi: 10.1242/dev.128.6.943. [DOI] [PMC free article] [PubMed] [Google Scholar]