Figure 1.
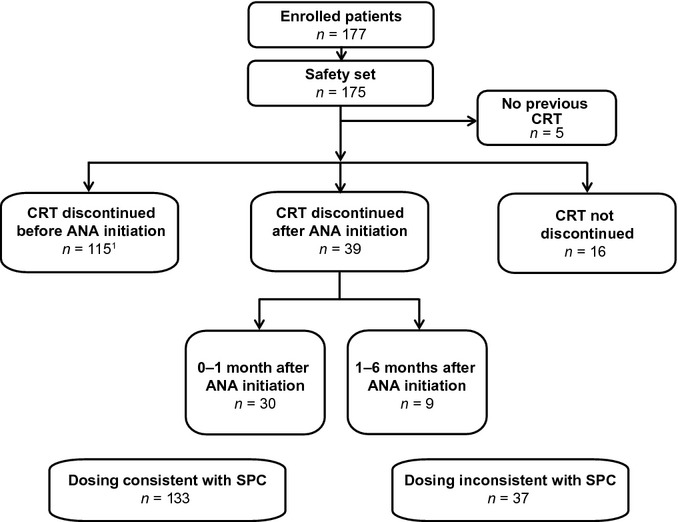
Patient disposition. ANA, anagrelide; CRT, cytoreductive therapy; SPC, Summary of Product Characteristics. 0–1 month: CRT was discontinued within the first 30 d of anagrelide treatment (median 10 d). 1–6 months: CRT was continued for ≥30 d (median 65 d), but was discontinued before anagrelide was discontinued or the patient completed the study period. Not discontinued: all other cases (i.e., patients received both their current CRT and anagrelide throughout the 6-month follow-up period). 1Includes two patients who restarted CRT after anagrelide initiation that was discontinued again in the 6-month follow-up period.
