Abstract
Background and Aim
Endocytoscopy (EC) at ultra-high magnification enables in vivo visualization of cellular atypia of gastrointestinal mucosae. Clear images are essential for precise diagnosis by EC. The aim of the present study was to evaluate the optimal staining method for EC in the colon.
Methods
Thirty prospectively enrolled patients were allocated 1:1:1 to three distinct staining methods: 0.05% crystal violet (CV) alone, 1% methylene blue (MB) alone, or CV + MB (CM double). Normal rectal mucosae were stained with each dye and videos of EC images were recorded. Visibility of nuclei and gland formation after staining were evaluated as ‘recognizable’ or ‘not recognizable’. Time for each parameter to become ‘recognizable’ was measured, and the average times for the three staining regimens were compared.
Results
MB alone and CM double staining resulted in ‘recognizable’ (102 ± 27 vs 89 ± 22 s, P = 0.263) nuclei within comparable periods of time, whereas CV alone was unable to identify nuclei. Gland formation became ‘recognizable’ sooner after CM double staining than after MB alone (61 ± 16 vs 108 ± 24 s, P < 0.001).
Conclusions
Double staining with CV and MB, which rapidly provided recognizable images of both nuclei and gland formation, is an appropriate staining regimen for colonic EC.
Keywords: crystal violet, endocytoscopy, methylene blue, staining regimen, ultra-high magnification
Introduction
Endocytoscopy (EC) is an emerging ultra-high magnifying technique enabling in vivo visualization of both structural and cellular atypia during endoscopic examination.1,2 Ultra-high magnifying endoscopy has been used to assess colorectal lesions.3 EC was able to differentiate non-neoplastic from neoplastic colonic lesions and to distinguish invasive cancer from adenoma.4,5
Features recognizable from EC include the shape of nuclei and the morphology of gland duct lumens in the superficial epithelial layer.5 Appropriate staining before EC observation is indispensable to obtain images similar to conventional pathological images obtained by staining with hematoxylin and eosin.6,7 We routinely use a mixture of 0.05% crystal violet (CV) and 1% methylene blue (MB) for colonic EC.5 Several studies have evaluated EC staining methods but these methods have not yet been standardized for colonic EC. For example, MB alone has been widely used for staining,7–10 whereas double staining with CV and MB has been reported useful for esophageal EC.11 Although an ex vivo study assessed the optimal staining conditions for EC,12 that study was carried out on a limited number of resected porcine colons.
Staining regimens for human colonic EC should allow the visualization of the shape of nuclei and gland duct lumens more clearly and rapidly. We therefore carried out a prospective, in vivo pilot study comparing three staining regimens, 0.05% CV, 1% MB, and the two together, to determine the appropriate staining conditions for human colonic EC.
Methods
Patients
The present study was carried out at Showa University Northern Yokohama Hospital, a tertiary referral center in Japan, from May 2011 to March 2012. Consecutive patients with indications for screening, surveillance, or diagnostic colonoscopy were recruited. Patients who showed evidence of rectal polyps on integrated-type EC were considered eligible for inclusion. Patients with inflammatory bowel disease were excluded. All patients underwent total bowel irrigation with 2 L polyethylene glycol solution, with additional polyethylene glycol given as necessary. Intravenous diazepam and butylscopolamine were given for sedation and prevention of peristalsis.
This study protocol was approved by the ethics committee of Showa University Northern Yokohama Hospital (No1201-05) and registered in UMIN Clinical Trials Registry (UMIN000007890). All participants provided written informed consent, and the study was conducted according to the principles of the Declaration of Helsinki.
Endocytoscopy
All colonoscopic examinations were carried out by highly trained endoscopists, each of whom had done more than 50 EC and more than 1500 colonoscopies, using an integrated-type EC (CF Y-0020-I, prototype; Olympus, Tokyo, Japan). This instrument has a single lens on its tip with a hand lever enabling the magnifying power of conventional endoscopic images to be increased to an ultra-high magnification power of ×380, covering 700 × 600-μm areas of tissue. EC can visualize the shape of nuclei and gland duct lumens in the epithelial superficial layer at a focus depth of 50 μm. A typical clear EC image of stained normal mucosa shows a regular pattern of uniformly sized fusiform nuclei and roundish lumens (Fig. 1).
Figure 1.
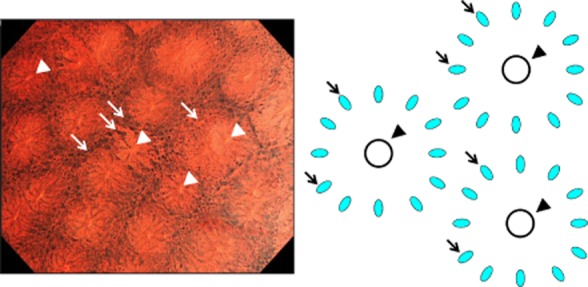
(Left panel) Endocytoscopic image and (right panel) scheme of normal rectal mucosae stained with 0.05% crystal violet and 1% methylene blue. Arrows show nuclei and arrowheads show gland formation.
Study procedure
Patients included in this study were allocated, in turn, to three distinct staining methods: 0.05% CV alone, 1% MB alone, or 0.05% CV plus 1% MB (CM double). Each dye (5 mL) was sprayed onto the surface of a rectal polyp and its surrounding normal mucosa through a non-traumatic catheter (Olympus 6233064; Olympus). The mucosal surface was immediately washed with distilled water containing dimethicone, followed by EC of the normal mucosa for 5 min, while keeping the tip of the EC in gentle contact with the surface of the mucosa. Images obtained throughout the procedure were stored on a digital versatile disk (DVD).
Image analysis
Visibility of nuclei and gland formation were evaluated as ‘recognizable’ or ‘not recognizable’. We considered nuclei and gland formation to be ‘recognizable’ when more than 50% of nuclei and gland formation were clearly visible in the field of view. To determine the optimal staining regimen, one endoscopist (K.I.), not on site and blinded to the staining regimen, assessed the times at which each specimen became ‘recognizable’ on DVD. The average time to visualization was calculated for each staining method, and the three times were compared.
In addition, to assess the reliability of this method, inter- and intraobserver agreements on the clarity of EC images of normal mucosae were assessed by three endoscopists (K.W., Y.M., and K.I.). Each evaluated 50 randomly selected EC images; 2 weeks later, the same images were again randomly presented to the three endoscopists for assessment.
After finding that CM double staining was optimal, we carried out a substudy in which colonic neoplastic lesions were stained with CM double to assess its usefulness and to compare results obtained for normal rectal mucosae and neoplastic lesions. We used the data of CM double obtained in the main study for normal mucosae. Ten adenomatous colon polyps of size ≥5 mm were stained with CM double and the time at which each specimen became ‘recognizable’ by EC was evaluated. The polyps were subsequently resected endoscopically and evaluated histopathologically by a single pathologist (S.H.) using World Health Organization criteria.13
Statistical analysis
SPSS 13.0 statistical software package (SPSS, Chicago, IL, USA) was used for data recording and analysis. Results were analyzed by Studen’s t-test or one-way analysis of variance (anova) with Tukey post-hoc analysis with a 5% level of significance. To determine inter- and intraobserver agreement, the proportion of agreement and weighted Cohen κ coefficient were determined (strengths of agreement of 0.01–0.2, 0.21–0.4, 0.41–0.6, 0.61–0.8, and 0.81–1.0 were considered poor, fair, moderate, good, and almost perfect, respectively).14 Using the same method as in the studies,15–18 we calculated the value of kappa for pairs of three readers and then computed an average kappa for all possible pairs. All results were expressed as mean ± standard deviation.
Results
Figure 2 shows the patient flowchart. From May 2011 to March 2012, 30 patients (24 males and six females, mean age 65 ± 11 years) were allocated equally to the three groups as follows. Ten (eight males, two females, mean age 65 ± 12 years) were assessed with CV alone, 10 (seven males, three females, mean age 61 ± 9 years) were assessed with MB alone, and 10 (nine males, one female, mean age 70 ± 10 years) were assessed with CM double. EC images were obtained for all patients. None experienced EC complications or adverse reactions.
Figure 2.
Patient flowchart. CV, crystal violet; MB, methylene blue; CM, mixture of crystal violet and methylene blue.
Figure 3 shows the times at which staining of nuclei and gland formation became ‘recognizable’. Figure 4 shows examples of each type of EC image. CV staining alone was unable to visualize nuclei at any exposure time. In contrast, MB alone and CM double were able to stain nuclei, with MB requiring a mean of 102 ± 22 s to reach ‘recognizable’, and CM double requiring 89 ± 27 s. The times required to reach ‘recognizable’ (P = 0.263) were statistically comparable.
Figure 3.
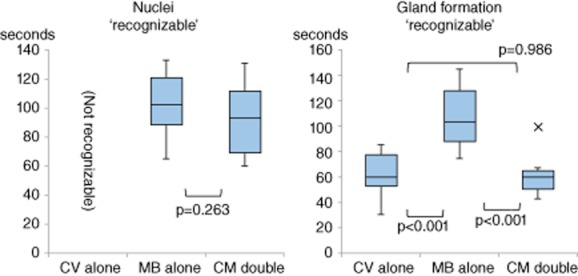
Average times for staining of nuclei and gland formation to reach ‘recognizable’. Bottom and top of boxes are lower and upper quartiles, respectively, and the band of the box is the median. Lines on the end of the whiskers are minimum and maximum, respectively. X denotes an outlier. The y-axis represents average time. CV, crystal violet; MB, methylene blue; CM, mixture of crystal violet and methylene blue.
Figure 4.
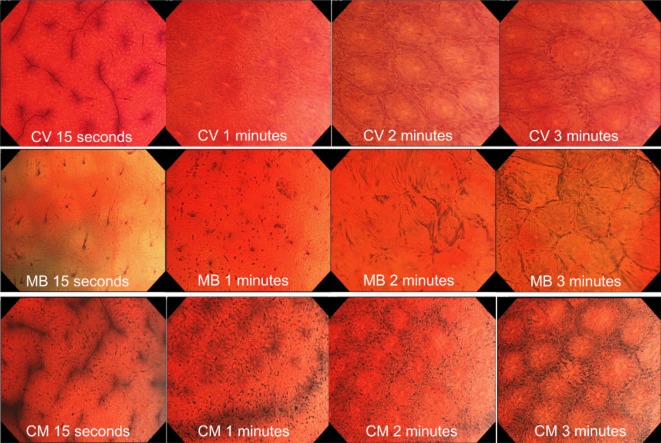
Typical examples of endocytoscopic images stained with 0.05% crystal violet (CV) alone, 1% methylene blue (MB) alone and CV plus MB (CM double). With CV alone, nuclei were not recognized and gland formation reached ‘recognizable’ in 1 min. With MB alone, both nuclei and gland formation reached ‘recognizable’ in 2 min. With CM double, both nuclei and gland formation reached ‘recognizable’ in 1 min.
All three staining methods were able to visualize gland formation, with CV alone, MB alone and CM double requiring 62 ± 17, 108 ± 24 and 61 ± 16 s, respectively, to reach ‘recognizable’. Compared with MB alone, the times required for CV alone (P < 0.001) and CM double (P < 0.001) to reach ‘recognizable’ were significantly shorter.
Comparing the time required for MB alone to stain glands (89 ± 27 s) and the time required for CM double to stain nuclei (108 ± 24 s), a significant difference (P = 0.0453) was observed. Taken together, the results for visualization of nuclei and gland formation indicate that the most appropriate dye for EC was CM double. Moreover, there was moderate inter- and intraobserver agreement, with kappa values of 0.412 and 0.502, respectively.
In a substudy, 10 consecutive patients (nine males, one female, mean age 59 ± 11 years), each with one colon polyp, were evaluated by EC after CM double staining. Mean lesion size was 6.7 ± 2.8 mm. All were histopathologically diagnosed as low-grade adenomas. The average times for the nuclei to reach ‘recognizable’ were 42 s (Fig. 5), being shorter for nuclei of neoplastic lesions than for normal rectal mucosae to reach ‘recognizable’ (42 ± 23 vs 89 ± 27 s, respectively, P < 0.001). In the identification of gland formation, the times to reach ‘recognizable’ were comparable (51 ± 18 vs 61 ± 16 s, respectively, P = 0.106).
Figure 5.
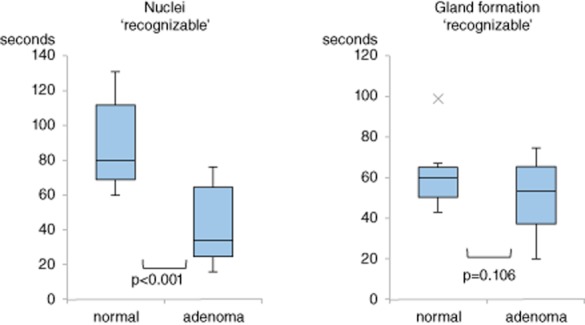
Average times for staining of nuclei and gland formation in normal rectal mucosa and low-grade adenoma to reach ‘recognizable’. Bottom and top of boxes are lower and upper quartiles, respectively, and the band of the box is the median. Lines on the end of the whiskers are minimum and maximum, respectively. X denotes an outlier. The y-axis represents average time.
Discussion
The main purpose of EC is the precise diagnosis of alimentary lesions. EC enables microscopic observation at the cellular level and assessment of both structural and cellular atypia in vivo. Real-time images of nuclei and gland formation obtained in vivo by EC are almost comparable to microscopic images obtained after hematoxylin and eosin staining of horizontal cross-sections of resected specimens, with the correlation between EC and histological diagnosis being statistically significant.19 EC can also distinguish neoplastic from non-neoplastic lesions and invasive cancer from adenoma.5 Clear staining is necessary for these diagnoses, with consistently clear images required for EC to be adopted as a standard technique.
The present study yielded two important clinical results. First, CM double was more appropriate than CV alone or MB alone for colonic EC. Second, adenomatous lesions were stained much more rapidly than normal mucosae.
Of the dyes tested, both MB alone and CM double were acceptable for EC staining, although CM double was preferable. MB has been widely used for staining in colonic EC. Consistent with previous studies, we found that staining with MB revealed details of cell structure, including both nuclei and gland formation. The mechanism of staining with MB involves absorption of dye into surface cells, followed by staining of nuclei and cytoplasm.20,21
We found that CV alone was unable to stain nuclei clearly in all specimens, but was more rapid than MB alone in identifying gland formation. CV alone effectively provided clear contrast views of the lumen and cytoplasm on the superficial layer of epithelial cells.
Our findings indicate that double staining with CV and MB is optimal for recognizing both nuclei and gland formation. The addition of CV to MB resulted in more rapid detection of gland formation than MB alone. Furthermore, double staining resulted in a clear contrast between the lumen and the cytoplasm, resulting in more rapid identification of nuclei. One drawback of EC is its prolonged procedure time. EC is relatively hazardous to patients and may induce intestinal peristalsis, making shortening of the endoscopic examination very important. Double staining with CV and MB may shorten the procedure time. Moreover, dilution of MB with CV may better visualize lesions by providing less dark luminal conditions than MB alone.
We also found that CM double was useful for staining of neoplastic lesions. EC images of low-grade adenomas show regular patterns of fusiform or roundish nuclei and slit-like smooth lumens.5 We found that CM double resulted in more rapid recognition of both nuclei in low-grade adenomas than in normal mucosae. The speed of dye absorption may be faster in neoplastic lesions than in normal tissue,1 which suggests that reducing the quantity of dye may be possible for neoplastic lesions.
Although MB has been reported to cause DNA damage,22 none of our patients experienced any complications. Following EC observation, we thoroughly rinsed the dye from the rectal mucosa with water, reducing any complications resulting from staining with MB. Moreover, to our knowledge, there have been no studies showing a clear relationship between MB-induced DNA damage and carcinogenesis in vivo.
The present study had three major limitations. First, this study was a non-randomized controlled trial and sample size for each dye was rather small. Staining conditions may vary among patients and their condition. This study was only a pilot study, suggesting the need for more patients and for patient randomization. Second, we examined only normal epithelia and low-grade adenomas in the rectum. We usually use EC for observing inflammatory disease or neoplastic lesions, especially invasive carcinomas. The intensity of vital staining has been reported to differ in samples with normal, inflamed, non-invasive neoplastic and invasive neoplastic areas.23,24 Considering our results, it is expected that invasive neoplastic lesions are stained more rapidly than normal mucosae and low-grade adenomas, but further experiments would be required. Third, other dyes, such as toluidine blue, were not investigated. Although we compared CV alone, MB alone and CM double, further studies including toluidine blue are needed.
In conclusion, the present study clearly showed that double staining with 0.05% CV and 1% MB is an appropriate staining regimen for colonic EC. EC can enhance the diagnostic ability of gastrointestinal endoscopy and this staining regimen is optimal for EC.
Acknowledgments
We thank all members of the Digestive Disease Center, Showa University Northern Yokohama Hospital, for helping with this study.
Conflict of Interests
Authors declare no conflict of interests for this article. No financial support was provided.
References
- Cipolletta L, Bianco MA, Rotondano G, et al. Endocytoscopy can identify dysplasia in aberrant crypt foci of the colorectum: A prospective in vivo study. Endoscopy. 2009;41:129–132. doi: 10.1055/s-0028-1103452. [DOI] [PubMed] [Google Scholar]
- Inoue H, Sasajima K, Kaga M, et al. Endoscopic in vivo evaluation of tissue atypia in the esophagus using a newly designed integrated endocytoscope: A pilot trial. Endoscopy. 2006;38:891–895. doi: 10.1055/s-2006-944667. [DOI] [PubMed] [Google Scholar]
- Tada M, Nishimura S, Watanabe Y. A new method for the ultramagnifying observation of the colon mucosa. Kyoto Pref. Univ. Med. 1982;91:349–354. [Google Scholar]
- Mori Y, Kudo S, Ikehara N, et al. Comprehensive diagnostic ability of endocytoscopy compared with biopsy for colorectal neoplasms: A prospective randomized noninferiority trial. Endoscopy. 2013;45:98–105. doi: 10.1055/s-0032-1325932. [DOI] [PubMed] [Google Scholar]
- Kudo SE, Wakamura K, Ikehara N, Mori Y, Inoue H, Hamatani S. Diagnosis of colorectal lesions with a novel endocytoscopic classification – a pilot study. Endoscopy. 2011;43:869–875. doi: 10.1055/s-0030-1256663. [DOI] [PubMed] [Google Scholar]
- Inoue H, Kudo SE, Shiokawa A. Technology insight: Laser-scanning confocal microscopy and endocytoscopy for cellular observation of the gastrointestinal tract. Nat. Clin. Pract. Gastroenterol. Hepatol. 2005;2:31–37. doi: 10.1038/ncpgasthep0072. [DOI] [PubMed] [Google Scholar]
- Eberl T, Jechart G, Probst A, et al. Can an endocytoscope system (ECS) predict histology in neoplastic lesions? Endoscopy. 2007;39:497–501. doi: 10.1055/s-2007-966446. [DOI] [PubMed] [Google Scholar]
- Neumann H, Vieth M, Neurath MF, Atreya R. Endocytoscopy allows accurate in vivo differentiation of mucosal inflammatory cells in IBD: A pilot study. Inflamm. Bowel Dis. 2013;19:356–362. doi: 10.1002/ibd.23025. [DOI] [PubMed] [Google Scholar]
- Hosoe N, Kobayashi T, Kanai T, et al. In vivo visualization of trophozoites in patients with amoebic colitis by using a newly developed endocytoscope. Gastrointest. Endosc. 2010;72:643–646. doi: 10.1016/j.gie.2010.04.031. [DOI] [PubMed] [Google Scholar]
- Meroni E, Gatteschi B, Fasoli A, et al. Detection of tissue abnormalities in normal mucosa surrounding colorectal cancer using an endocytoscopy system. Endoscopy. 2007;39:369–370. doi: 10.1055/s-2007-966098. [DOI] [PubMed] [Google Scholar]
- Minami H, Inoue H, Yokoyama A, et al. Recent advancement of observing living cells in the esophagus using CM double staining: Endocytoscopic atypia classification. Dis. Esophagus. 2012;25:235–241. doi: 10.1111/j.1442-2050.2011.01241.x. [DOI] [PubMed] [Google Scholar]
- Kodashima S, Fujishiro M, Takubo K, et al. Ex-vivo study of high-magnification chromoendoscopy in the gastrointestinal tract to determine the optimal staining conditions for endocytoscopy. Endoscopy. 2006;38:1115–1121. doi: 10.1055/s-2006-944915. [DOI] [PubMed] [Google Scholar]
- Bosman FT, Carneiro F, Hruban RH, Theise ND. WHO Classification of Tumours of the Digestive System. 4th edn. Lyon, France: IARC; 2010. [Google Scholar]
- Bland JM, Altman DG. Statistical methods for assessing agreement between two methods of clinical measurement. Lancet. 1986;1:307–310. [PubMed] [Google Scholar]
- Kundel HL, Polansky M. Measurement of observer agreement. Radiology. 2003;228:303–308. doi: 10.1148/radiol.2282011860. [DOI] [PubMed] [Google Scholar]
- Taplin SH, Rutter CM, Elmore JG, Seger D, White D, Brenner RJ. Accuracy of screening mammography using single versus independent double interpretation. AJR Am. J. Roentgenol. 2000;174:1257–1262. doi: 10.2214/ajr.174.5.1741257. [DOI] [PubMed] [Google Scholar]
- Herman PG, Khan A, Kallman CE, Rojas KA, Carmody DP, Bodenheimer MM. Limited correlation of left ventricular end-diastolic pressure with radiographic assessment of pulmonary hemodynamics. Radiology. 1990;174:721–724. doi: 10.1148/radiology.174.3.2305055. [DOI] [PubMed] [Google Scholar]
- Epstein DM, Dalinka MK, Kaplan FS, Aronchick JM, Marinelli DL, Kundel HL. Observer variation in the detection of osteopenia. Skeletal Radiol. 1986;15:347–349. doi: 10.1007/BF00348859. [DOI] [PubMed] [Google Scholar]
- Sasajima K, Kudo SE, Inoue H, et al. Real-time in vivo virtual histology of colorectal lesions when using the endocytoscopy system. Gastrointest. Endosc. 2006;63:1010–1017. doi: 10.1016/j.gie.2006.01.021. [DOI] [PubMed] [Google Scholar]
- Canto MI. Methylene blue chromoendoscopy for Barrett’s esophagus: Coming soon to your GI unit? Gastrointest. Endosc. 2001;54:403–409. doi: 10.1067/mge.2001.117958. [DOI] [PubMed] [Google Scholar]
- Canto MI, Setrakian S, Willis J, et al. Methylene blue-directed biopsies improve detection of intestinal metaplasia and dysplasia in Barrett’s esophagus. Gastrointest. Endosc. 2000;51:560–568. doi: 10.1016/s0016-5107(00)70290-2. [DOI] [PubMed] [Google Scholar]
- Olliver JR, Wild CP, Sahay P, Dexter S, Hardie LJ. Chromoendoscopy with methylene blue and associated DNA damage in Barrett’s oesophagus. Lancet. 2003;362:373–374. doi: 10.1016/s0140-6736(03)14026-3. [DOI] [PubMed] [Google Scholar]
- Canto MI. Staining in gastrointestinal endoscopy: The basics. Endoscopy. 1999;31:479–486. doi: 10.1055/s-1999-8041. [DOI] [PubMed] [Google Scholar]
- Kida M, Kobayashi K, Saigenji K. Routine chromoendoscopy for gastrointestinal diseases: Indications revised. Endoscopy. 2003;35:590–596. doi: 10.1055/s-2003-40228. [DOI] [PubMed] [Google Scholar]