SUMMARY
Objective
To examine genetic testing preferences in families containing multiple individuals with epilepsy.
Methods
One hundred forty-three individuals with epilepsy and 165 biological relatives without epilepsy from families containing multiple affected individuals were surveyed using a self-administered questionnaire. Four genetic testing scenarios were presented, defined by penetrance (100% vs. 50%) and presence or absence of clinical utility. Potential predictors of genetic testing preferences were evaluated using generalized estimating equations with robust Poisson regression models. The influence of 21 potential testing motivations was also assessed.
Results
For the scenario with 100% penetrance and clinical utility, 85% of individuals with epilepsy and 74% of unaffected relatives responded that they would definitely or probably want genetic testing. For the scenario with 100% penetrance but without clinical utility, the proportions who responded they would want testing were significantly lower in both affected individuals (69%) and unaffected relatives (57%). Penetrance (100% vs. 50%) was not a significant predictor of genetic testing interest. The highest-ranking motivations for genetic testing were: the possibility that the results could improve health or healthcare, the potential to know if epilepsy in the family is caused by a gene, and the possibility of changing behavior or lifestyle to prevent seizures.
Significance
Interest in epilepsy genetic testing may be high in affected and unaffected individuals in families containing multiple individuals with epilepsy, especially when testing has implications for improving clinical care.
Keywords: epilepsy, epidemiology, genetics, genetic testing, patient preferences
INTRODUCTION
Genetic research on the epilepsies had led to a dramatic increase in the number of genes recognized to play a role in specific epilepsy syndromes,1,2 and genetic testing has now become a major focus of consideration in clinical practice.3–8 Understanding the genetic testing preferences of people with epilepsy and their family members is essential to provide effective and sensitive genetic testing services for epilepsy.
Patient preferences for genetic testing in the epilepsies have been examined very little in previous studies. Three studies have investigated the views of research participants on genetic testing and the return of individual results, all of them employing qualitative research methods. One of these examined the perceived risks and benefits of genetic testing from the perspective of affected and unaffected members of families containing multiple affected individuals.9 Two other studies examined the expectations of research participants with regard to receiving individual genetic research results10 and the acceptability of being invited to participate in additional studies on the basis of their molecular genetic findings.11 All three of these qualitative studies suggested that most research participants would opt for genetic testing if offered. However, quantitative research on a larger number of individuals is critical to elucidate the trends uncovered in these studies and improve generalizability of the findings.
We are surveying previous participants in epilepsy genetics research, to assess the psychosocial impact of having a personal or family history of epilepsy, beliefs about epilepsy genetics, and interest in genetic testing. Here we report results on genetic testing preferences and the factors that had an important influence on these preferences.
METHODS
Study Sample
The study sample consisted of individuals who previously participated in the Epilepsy Family Study of Columbia University (EFSCU),12–18 a long-term genetic study of epilepsy that began in the mid-1980s as a familial aggregation study and progressed into a genetic linkage study. In the original study, families were ascertained through physician referral and self-referral in response to advertisements through voluntary organizations and a study website. Families were eligible if they contained either a sibling pair or three or more individuals with epilepsy not attributed to identified structural or metabolic CNS insults, regardless of syndrome. 117 families were included, containing an average of four affected individuals per family.
Study Design and Data Collection
Individuals in the 117 families were eligible for the current study if they had participated in our previous research either by being interviewed or giving a blood sample, and were alive, currently 18–79 years of age, and able to complete a self-administered questionnaire in English. Among 1274 participants in our previous studies, 316 were excluded because in 2013 they were deceased (N=100), younger than 18 years (N=16) or older than 79 years (N=115), had previously requested no contact for future studies (N=32) or did not speak English (N=53). Among the remaining 958 individuals, 27 more were excluded in the course of data collection because they were found to have died or to be unable to participate due to disability, leaving 931 individuals in 113 families (median 6, range 1–40 eligible individuals per family). The present analysis was further restricted to individuals with epilepsy (N=332) and their biological relatives without epilepsy (N=459), yielding a final target sample of 791 individuals after excluding those married-in to the families.
Eligible individuals were asked to complete a self-administered questionnaire either online through an instrument implemented in Survey Monkey (Survey Monkey, Inc., Palo Alto, California, USA, www.surveymonkey.com) or on paper using a version mailed to the participant’s residence. All study procedures were approved by the Columbia University Medical Center Institutional Review Board.
Genetic Testing Preferences and Predictors
The survey contained several questions about participants’ previous experience with genetic testing, including whether they had ever had genetic testing (for any disorder), what they were tested for, the setting in which testing was done (ordered by your doctor, a research study, more than one source, unknown), whether they received results, and what the results were. It also described four different hypothetical scenarios for a genetic test in epilepsy, defined by penetrance (“EVERYONE with the genetic change develops epilepsy” vs. “ONLY HALF of those with the genetic change will develop epilepsy”) and clinical utility (“knowing you have the genetic change would improve your medical care (for example, by helping your doctor choose better treatments or reducing the need for other tests)” vs. “knowing you have the genetic change would have no effect on your medical care”). For each scenario, participants were asked whether they would want genetic testing, with five response categories: definitely no, probably no, probably yes, definitely yes, and don’t know. For analysis, the five response categories were collapsed into two: definitely yes/probably yes vs. definitely no/probably no/don’t know.
We examined eight potential predictors of genetic testing preferences: epilepsy history, age (<40, 40–59, ≥60 years), sex, having children, education (college graduate vs. less), religious affiliation, and genetic causal attribution of epilepsy (assessed by questions described below). In individuals with epilepsy, we also examined the possible effects of broad epilepsy syndrome (generalized vs. focal), and epilepsy “severity” as assessed by time since last seizure (<1 year vs. ≥1 year), and lifetime number of seizures (≤20, 21–100, >100). Epilepsy history and syndrome were defined by diagnoses from our original genetic study, based on epileptologist review of data from semi-structured interviews and medical records.12,18 Information on time since last seizure and lifetime number of seizures was obtained from the current survey.
Genetic causal attribution of epilepsy was assessed by two survey questions. The first of these asked, “In your opinion, how big a role has genetics had in causing epilepsy in your family?” with responses: none, small, medium, big. The second question asked, “In your opinion, what do you think the chances are that you have a change or mutation in a gene that affects risk for epilepsy?” with responses no, small, moderate, or high chance, or don’t know.
Potential Motivations for Genetic Testing
To assess motivations for genetic testing preferences, the survey included a list of 21 factors that “people might consider when they make a decision about genetic testing.” Participants were asked whether each factor would affect their desire to obtain a genetic test for epilepsy and were presented with five response categories for each factor: much less likely to want testing, somewhat less likely to want testing, no effect on my desire for testing, somewhat more likely to want testing, much more likely to want testing.
Statistical Analysis
We used McNemar’s test to examine the significance of differences in individuals’ responses to the four genetic testing scenarios. To evaluate associations of potential predictors with participation rates and genetic testing preferences, we estimated prevalence ratios with Poisson regression models with robust standard errors,19 using generalized estimating equations to account for non-independence resulting from the inclusion of multiple individuals per family. In the analysis of genetic testing preferences, the outcome was defined as a response of definitely/probably yes (vs. definitely/probably no or don’t know) for each scenario. The prevalence ratios estimated in the models were virtually identical to ratios of the crude percentages of the outcome in each category; hence in the tables we show only the percentages and the p-values obtained from the regression analysis. To evaluate potential confounding, we also carried out multivariate analyses with all of the potential predictors included in the model. All analyses were carried out separately within individuals with epilepsy and biological relatives without epilepsy.
To analyze the 21 potential genetic testing motivations, we first treated them as quantitative variables, scoring the five response categories for each factor from −2 to 2 (i.e., −2=much less likely to want testing, 2=much more likely to want testing). For each motivation, we used a 1-sample t-test to assess whether the mean score differed significantly from 0, using a Bonferroni correction for multiple testing (i.e., critical value of (α=0.05/21=0.002).
Statistical analyses were carried out using IBM SPSS Statistics (Version 21).
RESULTS
As of June 16, 2014, we had attempted contact with 757 of the 791 subjects in our target sample and had reached 521 (69%) of them. Among subjects reached by telephone, 448 (86%) agreed to participate, and 315 (70%) of those who agreed completed the survey for an overall participation rate of 40% (315/791) as of the current date. Our sample is predominantly (93%) white non-Hispanic. Among all participants, 55% are college graduates and 59% are women. The mean age of all participants is 52 years (SEM 0.79).
To evaluate the potential for selection bias, we examined the predictors of participation among all 791 eligible subjects in our target sample. Participation rates increased with advancing age (<40 years 28%, 40–59 years 41%, ≥60 years 52%, p<0.001), and were higher in women than in men (42% vs. 37%) (p=0.03), but did not differ by education (college graduates 45%, others 40%, p=0.68). Individuals with epilepsy were more likely to participate than their unaffected relatives (44% vs. 37%, p=0.002).
Only two participants (both affected) reported having previously had clinical genetic testing for epilepsy. One of them reported having been tested for “currently known epilepsy genes” with a negative result, and the other reported having had “research testing plus local hospital testing for cause of seizures,” without receiving any results. Nine percent of individuals with epilepsy and six percent of biological relatives without epilepsy reported having had genetic testing for epilepsy in a research study, referring to their participation in our research.
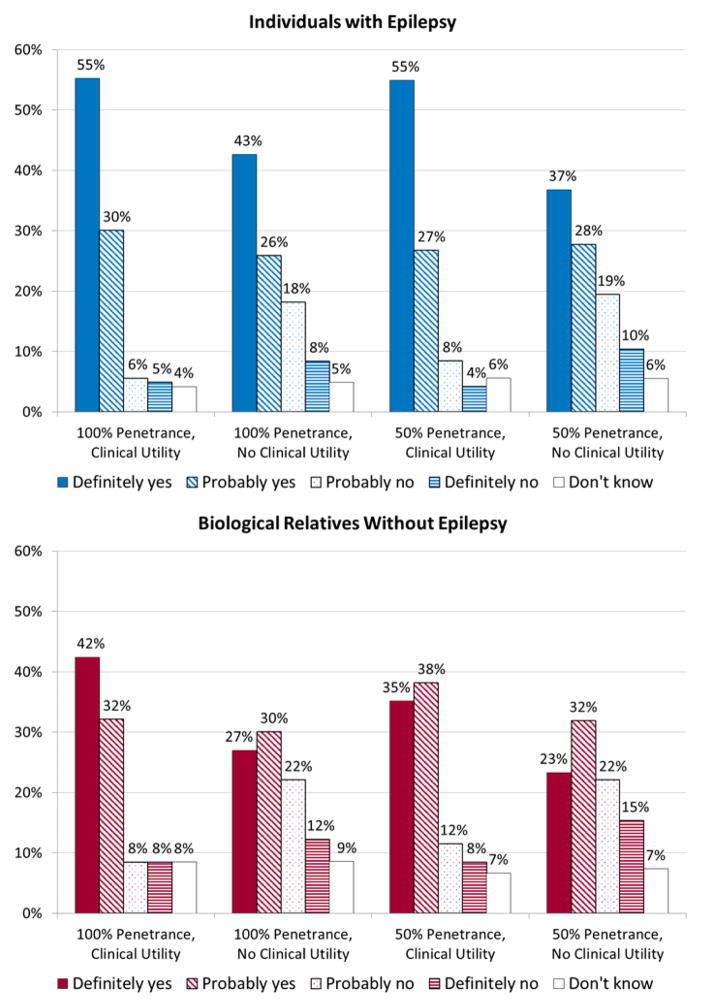
Among participants with epilepsy, 85% indicated that they would definitely or probably want genetic testing in the 100% penetrance, clinical utility scenario, but only 69% did so in the 100% penetrance, no clinical utility scenario (Figure 1). These proportions were very similar in the 50% penetrance scenarios (test with clinical utility 82%, without clinical utility 65%). The results from McNemar’s test indicated a significant difference between responses for a test with vs. without clinical utility in both the 100% penetrance and 50% scenarios (p<0.001 in each case). However, responses did not differ significantly between tests for genes with 100% vs. 50% penetrance, regardless of clinical utility.
Figure 1.
Distribution of responses to the four genetic testing scenarios by individuals with epilepsy and biological relatives without epilepsy.
Among biological relatives without epilepsy, fewer indicated they would definitely or probably want genetic testing than among individuals with epilepsy in all four scenarios. This difference was primarily attributed to a lower proportion of unaffected individuals who responded “definitely yes” (Figure 1). In comparisons between affected and unaffected individuals using dichotomized responses (definitely/probably yes vs. other), differences were significant for the 100% penetrance, clinical utility scenario (p=0.019) and the 100% penetrance, no clinical utility scenario (p=0.042) (robust Poisson regression with generalized estimating equations). As in affected individuals, the proportions of unaffected relatives who responded definitely/probably yes were significantly greater for a test with vs. without clinical utility, regardless of penetrance (p<0.001 in both cases), but did not differ between tests for genes with 100% vs. 50% penetrance.
Predictors of Genetic Testing Preferences
We first evaluated associations of predictors with responses to the 100% penetrance, clinical utility scenario (Table 1). Among participants with epilepsy, women were more likely than men to say they would definitely or probably want testing (93.8 vs. 71.2%, p=0.001). We found no significant differences among affected individuals with regard to other demographic variables (age, having children, education, or religion) or measures of genetic causal attribution. Individuals who reported a history of ≥100 seizures in their lifetimes were significantly more likely to want testing than were those with fewer seizures.
Table 1.
Proportion of individuals who would “definitely” or “probably” want to have genetic testing in a scenario with 100% penetrance and clinical utility, by demographic, genetic attribution, and epilepsy-related variables
| Predictors | Individuals with epilepsy | Biological relatives without epilepsy | ||||
|---|---|---|---|---|---|---|
|
| ||||||
| Na | % Definitely/probably yes | p-valueb | Na | % Definitely/probably yes | p-valueb | |
| All participants | 143 | 85.0 | -- | 165 | 74.2 | -- |
| Female | 87 | 93.8 | <0.001 | 95 | 72.2 | 0.74 |
| Male | 56 | 71.2 | Ref. | 70 | 76.9 | Ref. |
| Age <40 years | 28 | 92.9 | 0.27 | 38 | 81.6 | 0.14 |
| Age 40–59 years | 69 | 82.6 | 0.77 | 74 | 74.3 | 0.55 |
| Age ≥60 years | 46 | 84.8 | Ref. | 53 | 69.8 | Ref. |
| Do not have children | 49 | 89.8 | Ref. | 40 | 75.0 | Ref. |
| Have children | 91 | 82.4 | 0.24 | 123 | 75.6 | 0.93 |
| College graduate or higher | 77 | 84.0 | 0.75 | 92 | 81.3 | 0.11 |
| Less than college graduate | 65 | 86.0 | Ref. | 73 | 64.1 | Ref. |
| Catholic | 29 | 80.8 | 0.41 | 54 | 64.3 | 0.25 |
| Protestant | 48 | 88.9 | 0.78 | 49 | 81.0 | 0.59 |
| Otherc | 29 | 82.1 | 0.44 | 30 | 78.6 | 1.00 |
| None/Atheist/Prefer not to say | 29 | 88.9 | Ref. | 30 | 78.6 | Ref. |
| Opinion of role of genetics in causing epilepsy in familyd | ||||||
| Medium/big | 108 | 87.6 | 0.17 | 112 | 78.0 | 0.39 |
| None/small | 33 | 76.5 | Ref. | 45 | 68.4 | Ref. |
| Opinion of chance has a mutationd | ||||||
| Moderate/high | 78 | 88.7 | 0.15 | 64 | 86.9 | 0.001 |
| None/small/don’t know | 62 | 81.4 | Ref. | 101 | 66.0 | Ref. |
| Focal epilepsy | 80 | 87.5 | 0.29 | |||
| Generalized epilepsy | 34 | 79.4 | Ref. | |||
| Last seizure <1 year ago | 35 | 91.4 | 0.21 | |||
| Last seizure ≥1 year ago | 84 | 84.5 | Ref. | |||
| Lifetime number of seizures | ||||||
| ≤20 | 63 | 82.5 | Ref. | |||
| 21–100 | 24 | 79.2 | 0.72 | |||
| >100 | 29 | 96.6 | 0.02 | |||
| Unknown | 27 | 85.2 | 0.73 | |||
Total Ns vary among different variables because of missing data.
p-value from robust Poisson regression with generalized estimating equations, compared with reference group (denoted by Ref.).
Affected individuals: 10 identified as Jewish, 1 Hindu, 1 Buddhist, and 17 “Other.” Unaffected individuals: 6 identified as Jewish, 1 Buddhist, and 23 “Other.”
Genetic attribution assessed by responses to the questions, “In your opinion, how big a role has genetics had in causing the epilepsy in your family?” and “In your opinion, what do you think the chances are that you have a change or mutation in a gene that affects risk for epilepsy?”
Among unaffected relatives, neither sex nor any other demographic variable was significantly associated with testing preferences. However, unaffected relatives who believed they had a “moderate” or “high” chance of having an epilepsy-related mutation were significantly more likely to say they would definitely or probably want testing than were others (86.9 vs. 66.0%, p=0.001).
The results were essentially unchanged when all of the potential predictors were analyzed in multivariate models, separately within affected and unaffected individuals. In a parallel analysis of responses to the testing scenario with 100% penetrance and no clinical utility, the pattern of associations was the same, with the exception that the effect of education in unaffected individuals was significant (p=0.03). We did not analyze associations of potential predictors with responses to the 50% penetrance scenarios, since the responses to these questions did not differ from those in the 100% penetrance scenarios (Figure 1).
We were surprised that genetic testing preferences appeared to be unrelated to age or having children; hence we examined the possibility of effect modification between these two variables (Table 2). Since many individuals in our sample had children with epilepsy (a consequence of the way subjects were ascertained in our original genetic study), we also explored the impact of having affected children on responses in each age group. Among participants aged <40 years, the proportion who said they would definitely or probably want testing was greater in those with children than in those without. This was true in both affected and unaffected individuals, though it was significant only in unaffected individuals. Among individuals aged 40–59, testing interest was not significantly associated with having children overall, but participants who had affected children were more likely to say they would want testing than those had no affected children. Among unaffected individuals aged 40–59, all of those with affected children said they would want testing, but only 64% of those without affected children did so (p=0.03, compared with those without children). In the oldest group of participants (aged ≥60 years), individuals with epilepsy were significantly less likely to want testing if they had children than if they did not, and this was attributed to a lower proportion who said they would want testing among those who had affected children (74%) than among those whose children were unaffected (86%) or who did not have children (100%).
Table 2.
Combined effects of age and reproductive history on interest in genetic testing in 100% penetrance and clinical utility scenario
| Age and Reproductive History | Individuals with epilepsy | Biological relatives without epilepsy | ||||
|---|---|---|---|---|---|---|
|
| ||||||
| N | % Definitely/probably yes | p-valuea | N | % Definitely/probably yes | p-valuea | |
| <40 years | ||||||
| No children | 15 | 86.7 | Ref. | 17 | 64.7 | Ref. |
| Have children | 13 | 100.0 | 0.16 | 21 | 95.2 | 0.009 |
| None affected | 10 | 100.0 | 0.16 | 19 | 100.0 | 0.002 |
| ≥1 affected | 3 | 100.0 | 0.16 | 2 | 50.0 | 0.72 |
| 40–59 years | ||||||
| No children | 22 | 86.4 | Ref. | 18 | 88.9 | Ref. |
| Have children | 45 | 80.0 | 0.50 | 55 | 70.9 | 0.08 |
| None affected | 33 | 75.8 | 0.33 | 45 | 64.4 | 0.03 |
| ≥1 affected | 12 | 91.7 | 0.61 | 10 | 100.0 | 0.17 |
| ≥60 years | ||||||
| No children | 12 | 100.0 | Ref. | 5 | 60.0 | Ref. |
| Have children | 33 | 78.8 | 0.007 | 47 | 72.3 | 0.62 |
| None affected | 14 | 85.7 | 0.16 | 22 | 72.7 | 0.62 |
| ≥1 affected | 19 | 73.7 | 0.02 | 25 | 72.0 | 0.64 |
p-value from robust Poisson regression with generalized estimating equations, compared with reference group (denoted by Ref.).
Genetic Testing Motivations
Among the 21 potential genetic testing motivation items assessed, 12 differed significantly from zero (no effect on desire for testing) in affected individuals, and 13 in unaffected individuals (Table 3). The five most important factors in both affected and unaffected individuals were: possibility that the results could improve your health or healthcare, potential to know if the epilepsy in your family was caused by a gene, possibility of changing your behavior or lifestyle to prevent seizures, possibility of learning if your children are at risk, and chance to know some of your personal genetic information. Four items had a significant negative effect on genetic testing interest: possible effect on insurance coverage, possible impact on career, possible impact on privacy, and concern that the test might not be accurate. The mean values and order of items were similar in affected and unaffected individuals.
Table 3.
Mean values for genetic testing motivation itemsa
| Motivation Items | Individuals with epilepsy | Biological relatives without epilepsy | ||
|---|---|---|---|---|
|
| ||||
| Nb | Mean (95% CI) | Nb | Mean (95% CI) | |
| Possibility that the results could improve your health or healthcare | 139 | 1.2 (1.08, 1.39)c | 161 | 1.0 (0.84, 1.16)c |
| Potential to know if the epilepsy in your family is caused by a gene | 140 | 1.2 (1.02, 1.37)c | 163 | 1.0 (0.87, 1.17)c |
| Possibility of changing your behavior or lifestyle to prevent seizures | 140 | 1.0 (0.85, 1.22)c | 158 | 0.9 (0.77, 1.09)c |
| Possibility of learning if your children are at risk | 138 | 0.9 (0.73, 1.11)c | 158 | 0.9 (0.71, 1.05)c |
| Chance to know some of your personal genetic information | 140 | 0.8 (0.66, 1.03)c | 160 | 0.7 (0.56, 0.89)c |
| Having test results to share with your doctors | 139 | 0.7 (0.52, 0.87)c | 160 | 0.6 (0.42, 0.73)c |
| Possibility of avoiding activities that might be risky if you have a seizure(such as driving or swimming) | 139 | 0.6 (0.36, 0.76)c | 160 | 0.7 (0.54, 0.87)c |
| Possible effect on your decisions about having children | 140 | 0.4 (0.25, 0.59)c | 159 | 0.3 (0.17, 0.51)c |
| Possible effect on your future plans | 141 | 0.3 (0.07, 0.44) | 161 | 0.4 (0.20, 0.51)c |
| Confidence in the tests accuracy | 139 | 0.2 (0.00, 0.39) | 160 | 0.2 (−0.01, 0.35) |
| The ways the testing experience will make you feel | 137 | 0.1 (−0.02, 0.30) | 158 | 0.1 (−0.03, 0.28) |
| The need to get your blood drawn for the test | 139 | 0.1 (−0.04, 0.24) | 161 | 0.1 (−0.06, 0.19) |
| Possible effect on your decisions about marriage | 139 | 0.0 (−0.11, 0.20) | 160 | 0.1 (0.00, 0.29) |
| The thought of how your family would react to the testing | 138 | 0.0 (−0.14, 0.11) | 159 | 0.1 (−0.04, 0.22) |
| The thought of how your partner would react to the testing | 139 | 0.0 (−0.16, 0.09) | 158 | 0.0 (−0.08, 0.17) |
| Your religious, cultural, and/or spiritual beliefs | 137 | −0.1 (−0.20, 0.04) | 158 | 0.0 (−0.13, 0.10) |
| Affordability of the test | 138 | −0.1 (−0.37, 0.09) | 160 | 0.2 (0.01, 0.40) |
| Concern that the test might not be accurate | 138 | −0.3 (−0.51, −0.19)c | 161 | −0.4 (−0.57, −0.25)c |
| Possible impact on your privacy | 139 | −0.4 (−0.62, −0.27)c | 161 | −0.4 (−0.59, −0.28)c |
| Possible impact on your career | 139 | −0.5 (−0.66, −0.32)c | 160 | −0.4 (−0.55, −0.24)c |
| Possible effect on your insurance coverage | 138 | −0.8 (−0.94, −0.57)c | 160 | −0.6 (−0.81, −0.46)c |
Respondents were asked to rate “how each of these factors would affect your desire to get a genetic test for epilepsy risk.” Items were scored on a 5-point Likert scale, where −2=much less likely to want testing, −1=somewhat less likely to want testing, 0=no effect on my desire for testing, 1=somewhat more likely to want testing, 2=much more likely to want testing. Data are presented in order of means in individuals with epilepsy.
Ns vary slightly because of missing data.
Significantly different from zero (one-sample t-test) after Bonferroni correction for 21 tests (critical value of 0.05/21 = 0.0024).
DISCUSSION
This is the first quantitative study of preferences and motivations for genetic testing in families containing multiple individuals with epilepsy. In the families we studied, interest in epilepsy genetic testing was high both among individuals with epilepsy and their unaffected biological relatives, though it was higher in individuals with epilepsy. Clinical utility was a significant predictor of genetic testing interest: 85% of affected individuals and 74% of unaffected relatives said they would definitely or probably want a test with clinical utility, and these proportions were only 64% and 56% for a test without clinical utility.
Very few participants in our study had actually had clinical genetic testing for epilepsy, although many believed they had, as a result of participation in our previous genetic research. In the process of enrollment into our previous studies, we devoted considerable effort to clarifying the distinction between research (in which no results would be offered) and clinical genetic testing; this was also explained in our informed consent documents. The frequent confusion of these concepts by research participants illustrates the difficulty in conveying the message clearly, and may also reflect the strong interest in testing by members of these families.
Interestingly, penetrance of the gene described in our testing scenarios (100% vs. 50%) had no effect on testing preferences. These results may have differed if we had provided scenarios with lower penetrance (e.g., 10% or 20%), which are more applicable for genes in complex epilepsies.20 Also, the concept of penetrance might not have been understood by survey respondents, though we believe it was described clearly in our survey questions (“EVERYONE with the genetic change develops epilepsy” vs. “ONLY HALF of those with the genetic change will develop epilepsy”).
We identified few significant predictors of genetic testing preferences in either affected individuals or unaffected biological relatives (Table 1). Among affected individuals, women were more likely to want testing than were men. This gender effect has also been observed in studies of genetic testing preferences in other disorders, though findings are inconsistent.21 Genetic testing preferences also appeared to be related to severity of epilepsy: the proportion who said they would want testing was significantly greater among individuals who reported a lifetime history of >100 seizures than in those who reported fewer. Similarly, affected individuals were more likely to want testing if their last seizure was within the last year (91%) vs. longer ago (85%), although this difference was not significant. Among biological relatives without epilepsy, one of our measures of genetic attribution, an individual’s perception of the likelihood he or she carried a mutation in an epilepsy-related gene, significantly predicted genetic testing preferences.
Testing preferences appeared to be shaped in complex ways by age, reproductive history, and having affected offspring. Among individuals younger than 40 years, but not among older individuals, having children was associated with an increased interest in genetic testing. Younger individuals are likely to have children at an age when risk of developing epilepsy is high, and few of them had affected children at the time of the study; hence it makes sense that they would be interested in testing to inform risk in their children. The “possibility of learning if your children are at risk” ranked fourth highest among all 21 testing motivations we assessed, in both affected and unaffected individuals.
Among individuals aged 40–59 years, testing interest was not associated with having children overall, but, as might be expected, was greater in individuals with affected children than in those whose children were unaffected. These individuals might have considered genetic information potentially useful to inform risk in their children and grandchildren, some of whom were still young enough to be at risk of developing epilepsy.
In the oldest age group (age ≥60 years), however, the proportion of individuals with epilepsy who said they would want testing was actually lower among those with affected children (74%) than among those whose children were unaffected (86%). In our conversations with older individuals during the recruitment process, many appeared to have reduced interest in testing because they thought their children could have testing themselves. In addition, we speculate that older individuals might have been less comfortable with testing in general, and deterred by their feelings about having transmitted susceptibility (e.g., guilt, worry, sadness).
We previously carried out a qualitative study of the impact of genetic information from the perspective of people with epilepsy and their unaffected family members, involving in-depth interviews with 40 individuals from the same study population described here.9 The findings were very similar to those in our current much larger, quantitative study: 86% of individuals with epilepsy and 83% of those without epilepsy said they would have genetic testing if it were offered to them. Respondents identified many potential benefits of genetic testing, including learning what caused epilepsy in their families, being better able to care and advocate for children at risk, reducing guilt and blame, providing an increased sense of control, and relieving anxiety. They also cited potential negative impacts of testing, including increased blame and guilt, increased stigma and discrimination, self-imposed limitations on life goals, and altered public conceptions of epilepsy. Although respondents believed genetic testing would be useful for informing their reproductive choices, they also expressed fear that it could lead to external pressures to modify those choices. We did not address all of these issues in the current study, but did find that four items had a significant negative impact on genetic testing interest: possible effect on insurance coverage, possible impact on career, possible impact on privacy, and concern that the test might not be accurate. Our survey includes a series of questions that address stigma and discrimination, which will be reported separately.
Our study has several limitations. We assessed only hypothetical genetic testing preferences. Findings in other disorders clearly indicate that stated preferences do not equate with actual uptake. For example, in early studies of Huntington disease, most individuals at risk expressed interest in presymptomatic genetic testing,22–26 but uptake rates have been <10%.27 Similarly, in a 2006 review of genetic testing in breast cancer,28 rates of hypothetical interest were higher than those of actual uptake, though uptake has clearly increased in recent years.
Our findings cannot be generalized to all individuals with epilepsy because it is focused on a special subgroup: members of families containing multiple affected individuals. Such families are uncommon; in our recent population-based study, only approximately 15% of incident epilepsy probands had ≥1 first-degree relative with epilepsy.29 Nevertheless, members of these families are important stakeholders for assessment of genetic testing preferences.
The sample for the current study is also selected in other ways that may be related to genetic testing preferences. First, we examined only individuals who had previously participated in our genetic research. This implies that they had a special interest in, and likely an unusually good understanding of genetic influences in epilepsy. Levels of genetic attribution are naturally expected to be increased among individuals in these families, compared with unselected individuals with epilepsy. However, the association we observed, in unaffected family members, between genetic testing interest and the belief that they are likely to have inherited a risk-raising mutation may also apply to individuals not selected in this way. Because of the way these families were identified in our previous research (primarily through contacts with voluntary organizations), participants had relatively high levels of education (55% were college graduates), and were ethnically homogeneous, limiting our ability to examine the relationship of these variables to genetic preferences and likely increasing the proportions who would want testing. Estimates of genetic testing interest among affected individuals might have been inflated because participation rates were higher in women than in men, and affected women were more likely than affected men to say they would want testing. The sample contains few younger individuals for whom reproductive issues are likely to be most salient, and this is exacerbated by the higher participation rates among older individuals. Also, most affected individuals in these families do not have severe forms of epilepsy – 71% had not had seizures within the last year, and 44% reported ≤20 seizures in their lifetimes. As our results show, interest in genetic testing is likely to be higher in families with severe epilepsies. Finally, the current sample size is relatively small for investigation of some of the predictors.
Despite these limitations, our findings suggest that interest in epilepsy genetic testing may be high, particularly when testing has implications for clinical care. Moreover, interest is not limited to tests with clinical utility: two of the highest-ranking motivations for genetic testing were “the potential to know if the epilepsy in your family is caused by a gene” and “the chance to know some of your personal genetic information,” reflecting high value placed on personal rather than clinical utility. Study of these issues in the wider population of individuals with epilepsy (unselected by family history or participation in genetic research) would be extremely important. Also, the development of methods that facilitate informed decision making among individuals who express interest in epilepsy genetic testing should be a priority.
Acknowledgments
This research was supported by NIH grants R01 NS078419 and P50 HG007257. We are grateful to the families who generously donated their time to participation in this research.
Footnotes
DISCLOSURE
Dr. Chung is a paid consultant to GeneDx. The other authors declare no conflict of interest. We confirm that we have read the Journal’s position on issues involved in ethical publication and affirm that this report is consistent with those guidelines.
References
- 1.Helbig I, Lowenstein DH. Genetics of the epilepsies: where are we and where are we going? Curr Opin Neurol. 2013;26:179–185. doi: 10.1097/WCO.0b013e32835ee6ff. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hildebrand MS, Dahl HH, Damiano JA, Smith RJ, Scheffer IE, Berkovic SF. Recent advances in the molecular genetics of epilepsy. J Med Genet. 2013;50:271–279. doi: 10.1136/jmedgenet-2012-101448. [DOI] [PubMed] [Google Scholar]
- 3.Ottman R, Hirose S, Jain S, et al. Genetic testing in the epilepsies--report of the ILAE Genetics Commission. Epilepsia. 2010;51:655–670. doi: 10.1111/j.1528-1167.2009.02429.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Pal DK, Pong AW, Chung WK. Genetic evaluation and counseling for epilepsy. Nat Rev Neurol. 2010;6:445–453. doi: 10.1038/nrneurol.2010.92. [DOI] [PubMed] [Google Scholar]
- 5.Pong AW, Pal DK, Chung WK. Developments in molecular genetic diagnostics: an update for the pediatric epilepsy specialist. Pediatr Neurol. 2011;44:317–327. doi: 10.1016/j.pediatrneurol.2011.01.017. [DOI] [PubMed] [Google Scholar]
- 6.Scheffer IE. Genetic testing in epilepsy: what should you be doing? Epilepsy Curr. 2011;11:107–111. doi: 10.5698/1535-7511-11.4.107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hirose S, Scheffer IE, Marini C, et al. SCN1A testing for epilepsy: Application in clinical practice. Epilepsia. 2013;54:946–952. doi: 10.1111/epi.12168. [DOI] [PubMed] [Google Scholar]
- 8.Poduri A, Sheidley BR, Shostak S, Ottman R. Genetic testing in the epilepsies-developments and dilemmas. Nat Rev Neurol. 2014;10:293–299. doi: 10.1038/nrneurol.2014.60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Shostak S, Zarhin D, Ottman R. What’s at stake? Genetic information from the perspective of people with epilepsy and their family members. Soc Sci Med. 2011;73:645–654. doi: 10.1016/j.socscimed.2011.06.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Namey EE, Beskow LM. Epilepsy patient-participants and genetic research results as “answers”. J Empir Res Hum Res Ethics. 2011;6:21–29. doi: 10.1525/jer.2011.6.4.21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Beskow LM, Linney KN, Radtke RA, Heinzen EL, Goldstein DB. Ethical challenges in genotype-driven research recruitment. Genome Res. 2010;20:705–709. doi: 10.1101/gr.104455.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ottman R, Hauser WA, Stallone L. Semistructured interview for seizure classification: agreement with physicians’ diagnoses. Epilepsia. 1990;31:110–115. doi: 10.1111/j.1528-1157.1990.tb05368.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ottman R, Susser M. Data collection strategies in genetic epidemiology: The Epilepsy Family Study of Columbia University. J Clin Epidemiol. 1992;45:721–727. doi: 10.1016/0895-4356(92)90049-s. [DOI] [PubMed] [Google Scholar]
- 14.Ottman R, Lee JH, Hauser WA, et al. Reliability of seizure classification using a semistructured interview. Neurology. 1993;43:2526–2530. doi: 10.1212/wnl.43.12.2526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ottman R, Lee JH, Risch N, Hauser WA, Susser M. Clinical indicators of genetic susceptibility to epilepsy. Epilepsia. 1996;37:353–361. doi: 10.1111/j.1528-1157.1996.tb00571.x. [DOI] [PubMed] [Google Scholar]
- 16.Ottman R, Annegers JF, Risch N, Hauser WA, Susser M. Relations of genetic and environmental factors in the etiology of epilepsy. Ann Neurol. 1996;39:442–449. doi: 10.1002/ana.410390406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ottman R, Lee JH, Hauser WA, Risch N. Are generalized and localization-related epilepsies genetically distinct? Arch Neurol. 1998;55:339–344. doi: 10.1001/archneur.55.3.339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ottman R, Berenson K, Barker-Cummings C. Recruitment of families for genetic studies of epilepsy. Epilepsia. 2005;46:290–297. doi: 10.1111/j.0013-9580.2005.41904.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Deddens JA, Petersen MR. Approaches for estimating prevalence ratios. Occup Environ Med. 2008;65:481, 501–486. doi: 10.1136/oem.2007.034777. [DOI] [PubMed] [Google Scholar]
- 20.Helbig I, Hodge SE, Ottman R. Familial cosegregation of rare genetic variants with disease in complex disorders. Eur J Hum Genet. 2013;21:444–450. doi: 10.1038/ejhg.2012.194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sweeny K, Ghane A, Legg AM, Huynh HP, Andrews SE. Predictors of genetic testing decisions: a systematic review and critique of the literature. J Genet Couns. 2014;23:263–288. doi: 10.1007/s10897-014-9712-9. [DOI] [PubMed] [Google Scholar]
- 22.Stern R, Eldridge R. Attitudes of patients and their relatives to Huntington’s disease. J Med Genet. 1975;12:217–223. doi: 10.1136/jmg.12.3.217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Barette J, Marsden CD. Attitudes of families to some aspects of Huntington’s chorea. Psychol Med. 1979;9:327–336. doi: 10.1017/s0033291700030841. [DOI] [PubMed] [Google Scholar]
- 24.Teltscher B, Polgar S. Objective knowledge about Huntington’s disease and attitudes towards predictive tests of persons at risk. J Med Genet. 1981;18:31–39. doi: 10.1136/jmg.18.1.31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tyler A, Harper PS. Attitudes of subjects at risk and their relatives towards genetic counselling in Huntington’s chorea. J Med Genet. 1983;20:179–188. doi: 10.1136/jmg.20.3.179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Meissen GJ, Berchek RL. Intended use of predictive testing by those at risk for Huntington disease. Am J Med Genet. 1987;26:283–293. doi: 10.1002/ajmg.1320260206. [DOI] [PubMed] [Google Scholar]
- 27.Krukenberg RC, Koller DL, Weaver DD, Dickerson JN, Quaid KA. Two decades of Huntington disease testing: patient’s demographics and reproductive choices. J Genet Couns. 2013;22:643–653. doi: 10.1007/s10897-013-9596-0. [DOI] [PubMed] [Google Scholar]
- 28.Ropka ME, Wenzel J, Phillips EK, Siadaty M, Philbrick JT. Uptake rates for breast cancer genetic testing: a systematic review. Cancer Epidemiol Biomarkers Prev. 2006;15:840–855. doi: 10.1158/1055-9965.EPI-05-0002. [DOI] [PubMed] [Google Scholar]
- 29.Peljto AL, Barker-Cummings C, Vasoli VM, et al. Familial risk of epilepsy: a population-based study. Brain. 2014;137:795–805. doi: 10.1093/brain/awt368. [DOI] [PMC free article] [PubMed] [Google Scholar]