Abstract
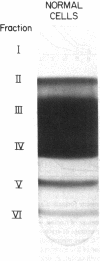
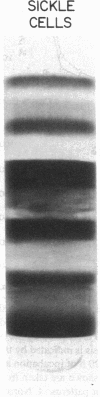
A large proportion of sickle erythrocytes is removed from the circulation by the macrophages of the reticuloendothelial system. In view of the proposed role for natural antibodies in the destruction of normal senescent erythrocytes, we looked for a possible similarity in the antibodies that bind in situ to senescent and sickle cells. Bound IgG molecules were detected by a highly sensitive rosetting antiglobulin test, using K562 myeloid cells. After separation on Stractan density gradients, the 0.6% most dense (senescent) normal cells and the most dense 40% sickle cells displayed membrane-bound IgG as reflected by the high proportion of rosettes formed. No antibody was found on low-density cells of either type. The bound antibodies were readily eluted from both sickle and normal senescent cells by carbohydrates containing alpha-galactosyl residues. These antibodies appear identical to the recently discovered human natural anti-alpha-galactosyl IgG (anti-Gal), an IgG antibody present in high titers in normal sera. Moreover, affinity-purified anti-Gal interacted specifically with sickle and normal cells depleted of the autologous antibodies. A similar pattern of binding to the various erythrocyte subpopulations was observed when the radiolabeled lectin with anti-alpha-galactosyl specificity, Bandeiraea simplicifolia, was used. In vitro phagocytosis of normal and sickle erythrocyte subpopulations correlated with the presence of anti-Gal on these cells. The in situ binding of anti-Gal to a large proportion of sickle erythrocytes may reflect an accelerated physiologic aging process by which immune recognition of prematurely exposed alpha-galactosyl-bearing antigenic sites contributes to shortened cell survival.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bauer T. W., Moore G. W., Hutchins G. M. The liver in sickle cell disease. A clinicopathologic study of 70 patients. Am J Med. 1980 Dec;69(6):833–837. doi: 10.1016/s0002-9343(80)80008-8. [DOI] [PubMed] [Google Scholar]
- Bensinger T. A., Gillette P. N. Hemolysis in sickle cell disease. Arch Intern Med. 1974 Apr;133(4):624–631. [PubMed] [Google Scholar]
- Bird G. W., Roy T. C. Human serum antibodies to melibiose and other carbohydrates. Vox Sang. 1980;38(3):169–171. doi: 10.1111/j.1423-0410.1980.tb02346.x. [DOI] [PubMed] [Google Scholar]
- Corash L., Shafer B., Perlow M. Heterogeneity of human whole blood platelet subpopulations. II. Use of a subhuman primate model to analyze the relationship between density and platelet age. Blood. 1978 Oct;52(4):726–734. [PubMed] [Google Scholar]
- Eckhardt A. E., Goldstein I. J. Isolation and characterization of a family of alpha-D-galactosyl-containing glycopeptides from Ehrlich ascites tumor cells. Biochemistry. 1983 Nov 8;22(23):5290–5297. doi: 10.1021/bi00292a007. [DOI] [PubMed] [Google Scholar]
- Galili U., Korkesh A., Kahane I., Rachmilewitz E. A. Demonstration of a natural antigalactosyl IgG antibody on thalassemic red blood cells. Blood. 1983 Jun;61(6):1258–1264. [PubMed] [Google Scholar]
- Galili U., Macher B. A., Buehler J., Shohet S. B. Human natural anti-alpha-galactosyl IgG. II. The specific recognition of alpha (1----3)-linked galactose residues. J Exp Med. 1985 Aug 1;162(2):573–582. doi: 10.1084/jem.162.2.573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galili U., Manny N., Izak G. EA rosette formation: a simple means to increase sensitivity of the antiglobulin test in patients with anti red cell antibodies. Br J Haematol. 1981 Feb;47(2):227–233. doi: 10.1111/j.1365-2141.1981.tb02783.x. [DOI] [PubMed] [Google Scholar]
- Galili U., Rachmilewitz E. A., Peleg A., Flechner I. A unique natural human IgG antibody with anti-alpha-galactosyl specificity. J Exp Med. 1984 Nov 1;160(5):1519–1531. doi: 10.1084/jem.160.5.1519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HARRIS J. W., BREWSTER H. H., HAM T. H., CASTLE W. B. Studies on the destruction of red blood cells. X. The biophysics and biology of sickle-cell disease. AMA Arch Intern Med. 1956 Feb;97(2):145–168. doi: 10.1001/archinte.1956.00250200021002. [DOI] [PubMed] [Google Scholar]
- Hayes C. E., Goldstein I. J. An alpha-D-galactosyl-binding lectin from Bandeiraea simplicifolia seeds. Isolation by affinity chromatography and characterization. J Biol Chem. 1974 Mar 25;249(6):1904–1914. [PubMed] [Google Scholar]
- Hebbel R. P., Miller W. J. Phagocytosis of sickle erythrocytes: immunologic and oxidative determinants of hemolytic anemia. Blood. 1984 Sep;64(3):733–741. [PubMed] [Google Scholar]
- Jeje O. M., Kelton J. G., Blajchman M. A. Quantitation of red cell membrane associated immunoglobulin in children with Plasmodium falciparum parasitaemia. Br J Haematol. 1983 Aug;54(4):567–572. doi: 10.1111/j.1365-2141.1983.tb02135.x. [DOI] [PubMed] [Google Scholar]
- Kay M. M., Goodman S. R., Sorensen K., Whitfield C. F., Wong P., Zaki L., Rudloff V. Senescent cell antigen is immunologically related to band 3. Proc Natl Acad Sci U S A. 1983 Mar;80(6):1631–1635. doi: 10.1073/pnas.80.6.1631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kay M. M. Mechanism of removal of senescent cells by human macrophages in situ. Proc Natl Acad Sci U S A. 1975 Sep;72(9):3521–3525. doi: 10.1073/pnas.72.9.3521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khansari N., Springer G. F., Merler E., Fudenberg H. H. Mechanisms for the removal of senescent human erythrocytes from circulation: specificity of the membrane-bound immunoglobulin G. Mech Ageing Dev. 1983 Jan;21(1):49–58. doi: 10.1016/0047-6374(83)90015-5. [DOI] [PubMed] [Google Scholar]
- Knyszynski A., Danon D., Kahane I., Rachmilewitz E. A. Phagocytosis of nucleated and mature beta thalassaemic red blood cells by mouse macrophages in vitro. Br J Haematol. 1979 Oct;43(2):251–255. doi: 10.1111/j.1365-2141.1979.tb03748.x. [DOI] [PubMed] [Google Scholar]
- Lalezari P., Jiang A. F., Kumar M., Lalezari I. Carbohydrate-specific antibodies in normal human sera. I. Characterization of specificity for beta-D-glucose. Vox Sang. 1984;47(2):133–145. doi: 10.1111/j.1423-0410.1984.tb01575.x. [DOI] [PubMed] [Google Scholar]
- Lutz H. U., Flepp R., Stringaro-Wipf G. Naturally occurring autoantibodies to exoplasmic and cryptic regions of band 3 protein, the major integral membrane protein of human red blood cells. J Immunol. 1984 Nov;133(5):2610–2618. [PubMed] [Google Scholar]
- Petz L. D., Yam P., Wilkinson L., Garratty G., Lubin B., Mentzer W. Increased IgG molecules bound to the surface of red blood cells of patients with sickle cell anemia. Blood. 1984 Jul;64(1):301–304. [PubMed] [Google Scholar]
- Phaire-Washington L., Wang E., Silverstein S. C. Phorbol myristate acetate stimulates pinocytosis and membrane spreading in mouse peritoneal macrophages. J Cell Biol. 1980 Aug;86(2):634–640. doi: 10.1083/jcb.86.2.634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piomelli S., Lurinsky G., Wasserman L. R. The mechanism of red cell aging. I. Relationship between cell age and specific gravity evaluated by ultracentrifugation in a discontinuous density gradient. J Lab Clin Med. 1967 Apr;69(4):659–674. [PubMed] [Google Scholar]
- Sharon R., Galili U. The rosetting antiglobulin test as a means of determining the D zygosity of human red cells. Vox Sang. 1984;46(3):175–179. doi: 10.1111/j.1423-0410.1984.tb00071.x. [DOI] [PubMed] [Google Scholar]
- Smalley C. E., Tucker E. M. Blood group A antigen site distribution and immunoglobulin binding in relation to red cell age. Br J Haematol. 1983 Jun;54(2):209–219. doi: 10.1111/j.1365-2141.1983.tb02089.x. [DOI] [PubMed] [Google Scholar]
- Spiro R. G., Bhoyroo V. D. Occurrence of alpha-D-galactosyl residues in the thyroglobulins from several species. Localization in the saccharide chains of the complex carbohydrate units. J Biol Chem. 1984 Aug 10;259(15):9858–9866. [PubMed] [Google Scholar]
- Suzuki E., Naiki M. Heterophile antibodies to rabbit erythrocytes in human sera and identification of the antigen as a glycolipid. J Biochem. 1984 Jan;95(1):103–108. doi: 10.1093/oxfordjournals.jbchem.a134571. [DOI] [PubMed] [Google Scholar]
- Takemura R., Werb Z. Secretory products of macrophages and their physiological functions. Am J Physiol. 1984 Jan;246(1 Pt 1):C1–C9. doi: 10.1152/ajpcell.1984.246.1.C1. [DOI] [PubMed] [Google Scholar]
- ten Brinke M., de Regt J. 51Cr-half life time of heavy and light human erythrocytes. Scand J Haematol. 1970;7(5):336–341. doi: 10.1111/j.1600-0609.1970.tb01911.x. [DOI] [PubMed] [Google Scholar]