Figure 6.
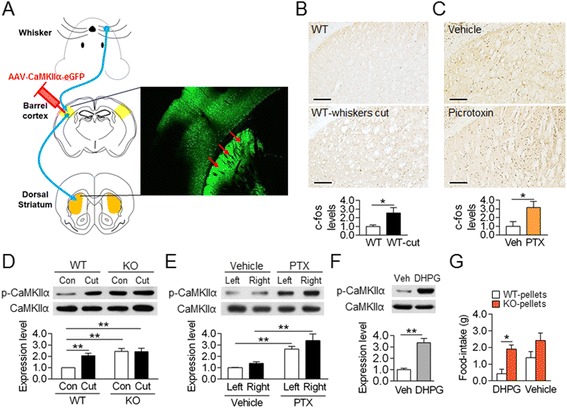
Whisker trimming increased the activity of the glutamatergic input from the barrel cortex into the dorsal striatum. (A) Schematic diagram showing the whisker sensory pathway heading, through the thalamus, to the barrel cortex, and the following corticostriatal projection. The corticostriatal projection was visualized by the injection of AAV2-CaMKIIα-eGFP into the barrel cortex. (B, C) Whisker trimming increased c-Fos induction in the dorsal striatum (B). Examined 24 h after whisker trimming. The infusion of picrotoxin in the barrel cortex increased c-Fos induction in the dorsal striatum in 1 h (C). Scale bar; 200 μm. (D, E) Whisker trimming in WT mice and the AC5 KO itself increased p-CaMKIIα in the dorsal striatum. p-CaMKIIα level was examined 24 h after whisker trimming (D). Infusion of picrotoxin in the barrel cortex increased the p-CaMKIIα level in the dorsal striatum in 1 h (E). Two-way ANOVA, Tukey’s HSD post hoc test; for whisker trimming (D), whiskers-cut [F (1, 28) = 7.243, p =0.0176], genotype [F (1, 28) = 24.43, p <0.001], and whiskers-cut × genotype interaction [F (1, 28) = 6.843, p = 0.0203]; for picrotoxin treatment (E), picrotoxin [F (1, 20) = 82.17, p <0.001], left-right [F (1, 20) = 7.830, p = 0.0111], and no picrotoxin × left-right interaction. (F) Stereotaxic injection of DHPG into the dorsal striatum increased the p-CaMKIIα level in 30 min. (G) Infusion of DHPG into the dorsal striatum through the pre-implanted cannulas in WT mice induced behavioral preferences for AC5 KO pellets over WT pellets. Two-way ANOVA, Tukey’s HSD test; DHPG [F (1, 24) = 4.520, p = 0.0440], food [F (1, 24) = 13.38, p = 0.0012], but no DHPG × food interaction. Data are presented as the mean ± SEM (n = 5-8). * and ** denote the difference between indicated groups at p <0.05 and p <0.01.
