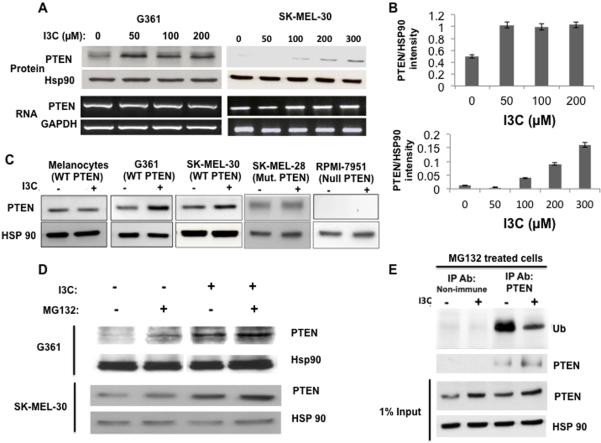
Figure 2.
I3C stimulates the level of wild PTEN protein by down-regulating ubiquitination and preventing the proteasomal degradation of PTEN. (A) G-361 and SK-MEL-30 melanoma cells were treated with the indicated concentrations of I3C for 48 hours. Isolated cell lysates were fractionated by SDS polyacrylamide gel electrophoresis and PTEN protein was monitored by western blot analysis in comparison the HSP90 gel loading control. Representative blots from three independent experiments are shown. Total cellular RNA was isolated, and PTEN transcript expression was determined by RT-PCR in comparison to the GAPDH constitutively expressed control transcript. The PCR products were visualized on a 1% agarose gel stained with ethidium bromide. Representative gels from three independent experiments are shown. (B) At each concentration of I3C, the relative levels of PTEN protein compared to Hsp90 was quantified by densitometry of the western blots shown in Fig 2A. The average protein band intensity from three independent experiments is displayed by bar graphs. (C) Human G-361 and SK-MEL-30 melanoma cells that express wild type PTEN, human RPMI melanoma cells with a null PTEN genotype, human SK-MEL-28 melanoma cells that express an A499G mutated PTEN and human primary epidermal melanocytes that express wild type PTEN were treated with or without 200 μM I3C for 48 hours. Total cell lysates were fractionated by SDS-polyacrylamide electrophoresis and the levels PTEN protein monitored by western blot analysis in comparison the HSP90 gel loading control. Representative blots from three independent experiments are shown. (D) G-361 and SK-MEL-30 melanoma cells were treated with or without I3C for 48 hours and each set of cells were incubated in the presence or absence of 10 μM MG-132, a 26S-specific proteasome inhibitor, for the last 5 hours of the incubation. PTEN protein was assessed in total cell lysates by western blot analysis in comparison to the HSP90 gel loading control. (E) G-361 cells were treated for 48 hours in the presence or absence of I3C, and the cells treated with 10 μM MG-132 for the last 5 hours of the incubation. Cell extracts were immunoprecipitated with either PTEN-specific or non-immune antibodies and western blots of electrophoretically fractionated samples probed with anti-ubiquitin or PTEN-specific antibodies in comparison to the HSP90 gel loading control. The total level of PTEN protein produced in each condition is shown by the western blot of the 1% input of cell extracts. For clarity of presentation, paired gel lanes of cells not treated with the MG132 26S-proteasome inhibitor were cropped from the upper panel of Fig 2D, which presents the western blot of ubiquitinated PTEN compared to the non-immune antibody control.