Figure 4.
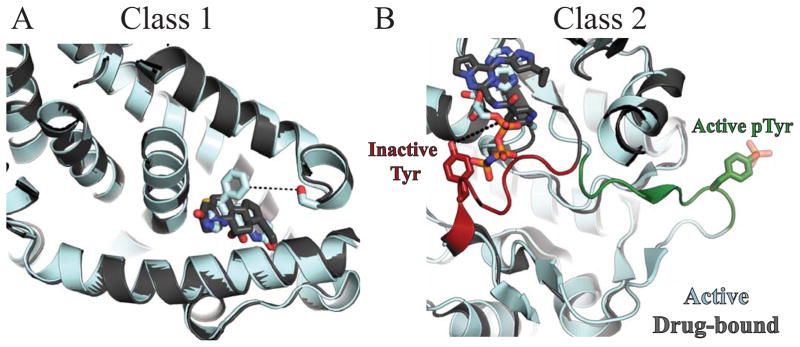
Structural views example Class 1 and Class 2 hits. Active targets are shown in cyan, inactive targets in grey. Small molecule ligands are shown in sticks. A: Overlapped structures of active (2AA2) and inactive (3VHV) forms of Class 1 target mineralocorticoid receptor. When phosphorylated, Ser843 (red) inactivates the receptor by preventing agonist binding. As shown, the inhibitor binds at the same site as the agonist. Inhibitor affinity would likely be reduced by phosphorylation of Ser843 6.4Å away (dashed lines). B: Overlapped structures of active, phopshorylated insulin receptor (1IR3) and inactive insulin-like growth factor 1 receptor (3NW7), a Class 2 target. The two proteins share 91.3% sequence similarity within the crystallized constructs and 100% sequence identity within the activation loop. In the inactive conformation, the activation loop is red. Tyr1161 (sticks) is 7.1Å away from the “DFG-out” inhibitor (dashed lines). In the active, phosphorylated conformation, the activation loop is green. Phosphorylation simultaneously activates insulin-like growth factor 1 receptor and can reduce inhibitor affinity.
