Abstract
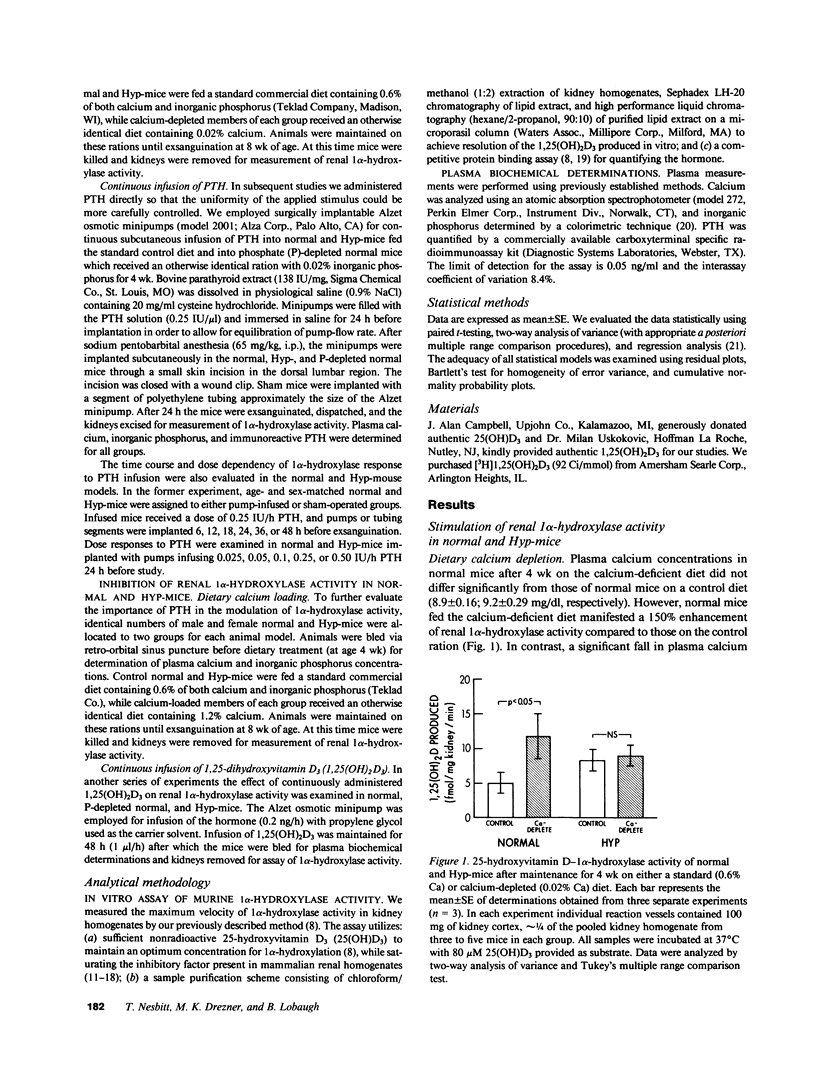
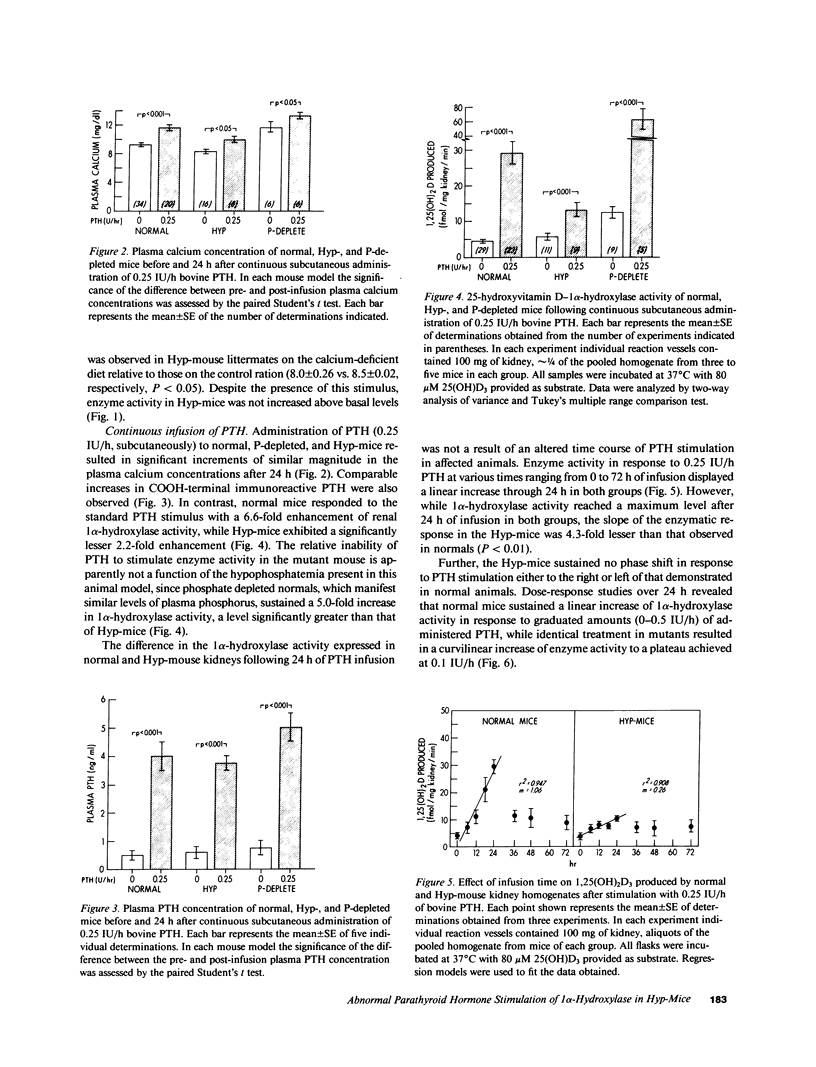
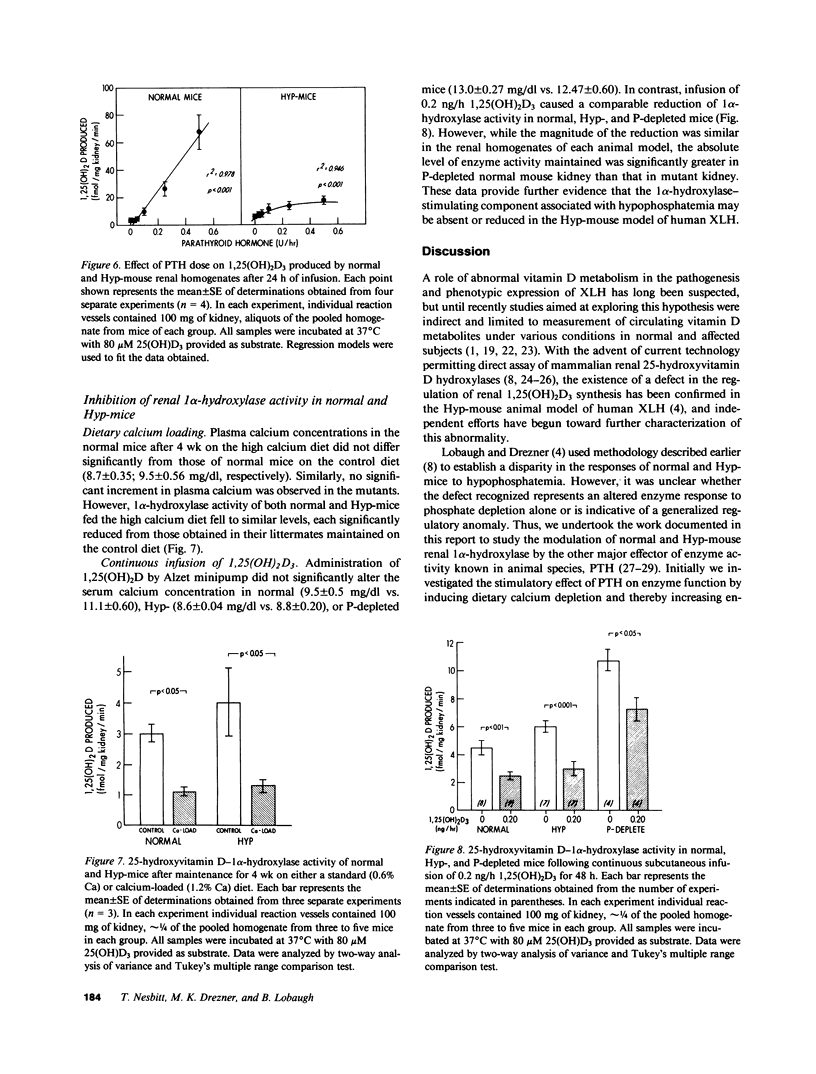
Abnormal regulation of vitamin D metabolism is a feature of X-linked hypophosphatemic rickets in man and of the murine homologue of the disease in the hypophosphatemic (Hyp)-mouse. We previously reported that mutant mice have abnormally low renal 25-hydroxyvitamin D-1 alpha-hydroxylase (1 alpha-hydroxylase) activity for the prevailing degree of hypophosphatemia. To further characterize this defect, we examined whether Hyp-mouse renal 1 alpha-hydroxylase activity responds normally to other stimulatory and inhibitory controls of enzyme function. We studied stimulation by parathyroid hormone (PTH) using: (a) a calcium-deficient (0.02% Ca) diet to raise endogenous PTH; or (b) 24-h continuous infusion of 0.25 IU/h bovine PTH via osmotic minipump. In both cases enzyme activity of identically treated normal mice increased to greater levels than those attained by Hyp-mice. The relative inability of PTH to stimulate 1 alpha-hydroxylase activity is not a function of the hypophosphatemia in the Hyp-mouse since PTH-infused, phosphate-depleted normal mice sustained a level of enzyme activity greater than that of normal and Hyp-mice. In further studies we investigated inhibition of enzyme activity by using: (a) a calcium-loaded (1.2% Ca) diet to suppress endogenous PTH; or (b) 24-h continuous infusion of 0.2 ng/h 1,25-dihydroxyvitamin D3 (1,25(OH)2D3). The 1 alpha-hydroxylase activity of normal and Hyp-mice was significantly reduced to similar absolute levels following maintenance on the calcium-loaded diet. Further, infusion of 1,25(OH)2D3 caused a comparable reduction of 1 alpha-hydroxylase activity in normal, Hyp-, and phosphate-depleted normal mice. These observations indicate that the inhibitory control of 1 alpha-hydroxylase by reduced levels of PTH or increased 1,25(OH)2D3 concentrations is intact in the mutants. However, the inability of PTH and hypophosphatemia to stimulate enzyme activity in a manner analogous to that in normal and phosphate-depleted mice indicates that a generalized defect of 1 alpha-hydroxylase regulation is manifest in Hyp-mice.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baksi S. N., Kenny A. D. Acute effect of estradiol on the renal vitamin D hydroxylases in Japanese quail. Biochem Pharmacol. 1978;27(24):2765–2768. doi: 10.1016/0006-2952(78)90187-9. [DOI] [PubMed] [Google Scholar]
- Botham K. M., Tanaka Y., DeLuca H. F. 25-Hydroxyvitamin D3-1-hydroxylase. Inhibition in vitro by rat and pig tissues. Biochemistry. 1974 Nov 19;13(24):4961–4966. doi: 10.1021/bi00721a014. [DOI] [PubMed] [Google Scholar]
- Chesney R. W., Mazess R. B., Rose P., Hamstra A. J., DeLuca H. F. Supranormal 25-hydroxyvitamin D and subnormal 1,25-dihydroxyvitamin D: their role in X-linked hypophosphatemic rickets. Am J Dis Child. 1980 Feb;134(2):140–143. doi: 10.1001/archpedi.1980.02130140014005. [DOI] [PubMed] [Google Scholar]
- Cloix J. F., Bachelet M., Ulmann A., Funck-Brentano J. L. 25-hydroxycholecalciferol-binding protein: partial purification from rat duodenal mucosa cells. Biochem Biophys Res Commun. 1978 Aug 29;83(4):1456–1463. doi: 10.1016/0006-291x(78)91384-0. [DOI] [PubMed] [Google Scholar]
- Cooke N. E., Walgate J., Haddad J. G., Jr Human serum binding protein for vitamin D and its metabolites. I. Physicochemical and immunological identification in human tissues. J Biol Chem. 1979 Jul 10;254(13):5958–5964. [PubMed] [Google Scholar]
- Cooke N. E., Walgate J., Haddad J. G., Jr Human serum binding protein for vitamin D and its metabolites. II. Specific, high affinity association with a protein in nucleated tissue. J Biol Chem. 1979 Jul 10;254(13):5965–5971. [PubMed] [Google Scholar]
- Cowgill L. D., Goldfarb S., Lau K., Slatopolsky E., Agus Z. S. Evidence for an intrinsic renal tubular defect in mice with genetic hypophosphatemic rickets. J Clin Invest. 1979 Jun;63(6):1203–1210. doi: 10.1172/JCI109415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DRYER R. L., TAMMES A. R., ROUTH J. I. The determination of phosphorus and phosphatase with N-phenyl-p-phenylenediamine. J Biol Chem. 1957 Mar;225(1):177–183. [PubMed] [Google Scholar]
- Eicher E. M., Southard J. L., Scriver C. R., Glorieux F. H. Hypophosphatemia: mouse model for human familial hypophosphatemic (vitamin D-resistant) rickets. Proc Natl Acad Sci U S A. 1976 Dec;73(12):4667–4671. doi: 10.1073/pnas.73.12.4667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fukase M., Avioli L. V., Birge S. J., Chase L. R. Abnormal regulation of 25-hydroxyvitamin D3-1 alpha-hydroxylase activity by calcium and calcitonin in renal cortex from hypophosphatemic (Hyp) mice. Endocrinology. 1984 Apr;114(4):1203–1207. doi: 10.1210/endo-114-4-1203. [DOI] [PubMed] [Google Scholar]
- Fukase M., Birge S. J., Jr, Rifas L., Avioli L. V., Chase L. R. Regulation of 25 hydroxyvitamin D3 1-hydroxylase in serum-free monolayer culture of mouse kidney. Endocrinology. 1982 Mar;110(3):1073–1075. doi: 10.1210/endo-110-3-1073. [DOI] [PubMed] [Google Scholar]
- Garabedian M., Holick M. F., Deluca H. F., Boyle I. T. Control of 25-hydroxycholecalciferol metabolism by parathyroid glands. Proc Natl Acad Sci U S A. 1972 Jul;69(7):1673–1676. doi: 10.1073/pnas.69.7.1673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haddad J. G., Birge S. J. Widespread, specific binding of 25-hydroxycholecalciferol in rat tissues. J Biol Chem. 1975 Jan 10;250(1):299–303. [PubMed] [Google Scholar]
- Henry H. L. Regulation of the hydroxylation of 25-hydroxyvitamin D3 in vivo and in primary cultures of chick kidney cells. J Biol Chem. 1979 Apr 25;254(8):2722–2729. [PubMed] [Google Scholar]
- Insogna K. L., Broadus A. E., Gertner J. M. Impaired phosphorus conservation and 1,25 dihydroxyvitamin D generation during phosphorus deprivation in familial hypophosphatemic rickets. J Clin Invest. 1983 Jun;71(6):1562–1569. doi: 10.1172/JCI110912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiebzak G. M., Roos B. A., Meyer R. A., Jr Secondary hyperparathyroidism in X-linked hypophosphatemic mice. Endocrinology. 1982 Aug;111(2):650–652. doi: 10.1210/endo-111-2-650. [DOI] [PubMed] [Google Scholar]
- Kream B. E., Reynolds R. D., Knutson J. C., Eisman J. A., DeLuca H. F. Intestinal cytosol binders of 1,25-dihydroxyvitamin D and 25-hydroxyvitamin D. Arch Biochem Biophys. 1976 Oct;176(2):779–787. doi: 10.1016/0003-9861(76)90222-8. [DOI] [PubMed] [Google Scholar]
- Lobaugh B., Drezner M. K. Abnormal regulation of renal 25-hydroxyvitamin D-1 alpha-hydroxylase activity in the X-linked hypophosphatemic mouse. J Clin Invest. 1983 Feb;71(2):400–403. doi: 10.1172/JCI110783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lobaugh B., Drezner M. K. Measurement of 25-hydroxyvitamin D-1 alpha-hydroxylase activity in mammalian kidney. Anal Biochem. 1983 Mar;129(2):416–424. doi: 10.1016/0003-2697(83)90571-7. [DOI] [PubMed] [Google Scholar]
- Lyles K. W., Clark A. G., Drezner M. K. Serum 1,25-dihydroxyvitamin D levels in subjects with X-linked hypophosphatemic rickets and osteomalacia. Calcif Tissue Int. 1982 Mar;34(2):125–130. doi: 10.1007/BF02411222. [DOI] [PubMed] [Google Scholar]
- Lyles K. W., Drezner M. K. Parathyroid hormone effects on serum 1,25-dihydroxyvitamin D levels in patients with X-linked hypophosphatemic rickets: evidence for abnormal 25-hydroxyvitamin D-1-hydroxylase activity. J Clin Endocrinol Metab. 1982 Mar;54(3):638–644. doi: 10.1210/jcem-54-3-638. [DOI] [PubMed] [Google Scholar]
- Meyer R. A., Jr, Gray R. W., Meyer M. H. Abnormal vitamin D metabolism in the X-linked hypophosphatemic mouse. Endocrinology. 1980 Nov;107(5):1577–1581. doi: 10.1210/endo-107-5-1577. [DOI] [PubMed] [Google Scholar]
- Meyer R. A., Jr, Gray R. W., Roos B. A., Kiebzak G. M. Increased plasma 1,25-dihydroxyvitamin D after low calcium challenge in X-linked hypophosphatemic mice. Endocrinology. 1982 Jul;111(1):174–177. doi: 10.1210/endo-111-1-174. [DOI] [PubMed] [Google Scholar]
- Obie J. F., Cooper C. W. Loss of calcemic effects of calcitonin and parathyroid hormone infused continuously into rats using the Alzet osmotic minipump. J Pharmacol Exp Ther. 1979 Jun;209(3):422–428. [PubMed] [Google Scholar]
- Podbesek R., Edouard C., Meunier P. J., Parsons J. A., Reeve J., Stevenson R. W., Zanelli J. M. Effects of two treatment regimes with synthetic human parathyroid hormone fragment on bone formation and the tissue balance of trabecular bone in greyhounds. Endocrinology. 1983 Mar;112(3):1000–1006. doi: 10.1210/endo-112-3-1000. [DOI] [PubMed] [Google Scholar]
- Posillico J. T., Lobaugh B., Muhlbaier L. H., Drezner M. K. Abnormal parathyroid function in the X-linked hypophosphatemic mouse. Calcif Tissue Int. 1985 Jul;37(4):418–422. doi: 10.1007/BF02553712. [DOI] [PubMed] [Google Scholar]
- Scriver C. R., Reade T. M., DeLuca H. F., Hamstra A. J. Serum 1,25-dihydroxyvitamin D levels in normal subjects and in patients with hereditary rickets or bone disease. N Engl J Med. 1978 Nov 2;299(18):976–979. doi: 10.1056/NEJM197811022991803. [DOI] [PubMed] [Google Scholar]
- Tam C. S., Jones G., Heersche J. N. The effect of vitamin D restriction and repletion on bone apposition in the rat and its dependence on parathyroid hormone. Endocrinology. 1981 Nov;109(5):1448–1453. doi: 10.1210/endo-109-5-1448. [DOI] [PubMed] [Google Scholar]
- Tanaka Y., DeLuca H. F. Measurement of mammalian 25-hydroxyvitamin D3 24R-and 1 alpha-hydroxylase. Proc Natl Acad Sci U S A. 1981 Jan;78(1):196–199. doi: 10.1073/pnas.78.1.196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tenenhouse H. S. Abnormal renal mitochondrial 25-hydroxyvitamin D3-1-hydroxylase activity in the vitamin D and calcium deficient X-linked Hyp mouse. Endocrinology. 1983 Aug;113(2):816–818. doi: 10.1210/endo-113-2-816. [DOI] [PubMed] [Google Scholar]
- Tenenhouse H. S. Investigation of the mechanism for abnormal renal 25-hydroxyvitamin D3-1-hydroxylase activity in the X-linked Hyp mouse. Endocrinology. 1984 Aug;115(2):634–639. doi: 10.1210/endo-115-2-634. [DOI] [PubMed] [Google Scholar]
- Tenenhouse H. S., Scriver C. R. Renal adaptation to phosphate deprivation in the Hyp mouse with X-linked hypophosphatemia. Can J Biochem. 1979 Jun;57(6):938–944. doi: 10.1139/o79-114. [DOI] [PubMed] [Google Scholar]
- Van Baelen H., Bouillon R., De Moor P. Binding of 25-hydroxycholecalciferol in tissues. J Biol Chem. 1977 Apr 25;252(8):2515–2518. [PubMed] [Google Scholar]
- Van Baelen H., Bouillon R., De Moor P. Vitamin D-binding protein (Gc-globulin) binds actin. J Biol Chem. 1980 Mar 25;255(6):2270–2272. [PubMed] [Google Scholar]
- Vieth R., Fraser D. Kinetic behavior of 25-hydroxyvitamin D-1-hydroxylase and -24-hydroxylase in rat kidney mitochondria. J Biol Chem. 1979 Dec 25;254(24):12455–12460. [PubMed] [Google Scholar]


